Home > Press > Study Shows That Size Affects Structure of Hollow Nanoparticles
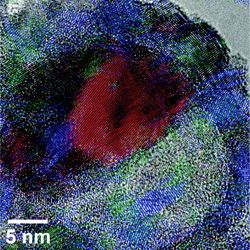 |
| Image of a half-oxidized 26 nanometer nanoparticle. The nickel region is colored red, and the nickel oxide is colored blue and green. Image courtesy of ACS Nano. |
Abstract:
A new study from North Carolina State University shows that size plays a key role in determining the structure of certain hollow nanoparticles. The researchers focused on nickel nanoparticles, which have interesting magnetic and catalytic properties that may have applications in fields as diverse as energy production and nanoelectronics.
By Matt Shipman, News Services
Study Shows That Size Affects Structure of Hollow Nanoparticles
Raleigh, NC | Posted on April 13th, 2010The principles we're uncovering here have great potential for nanofabrication - the creation of materials that have very small features, with many applications in fields ranging from electronics to medicine," says Dr. Joe Tracy, an assistant professor of materials science and engineering at NC State and co-author of the study. "This study improves our understanding of hollow nanoparticles and is a foundation for future work on applications in ultra-high density magnetic recording and more efficient catalysts, which is useful for chemical production, waste treatment and energy production."
At issue is the oxidation of nickel nanoparticles. If you start with a "core" piece of nickel and oxidize it, exposing it to oxygen at high temperatures, the structure of the material changes. If the material is partially oxidized - exposed to oxygen and high heat for a limited time - a solid nickel oxide shell forms around the material.
If the material is exposed to heat and oxygen for a longer period of time, further oxidation occurs. The external shell remains, but nickel is transported out of the core, leaving a void. If the material is fully oxidized, a larger void is created - leaving the nickel oxide shell effectively hollow. This conversion of solid to hollow nanoparticles is known as the "nanoscale Kirkendall Effect."
But what NC State researchers have found is that the size of the nickel core also plays a key role in the structure of these particles. For example, in smaller nickel nanoparticles - those with cores having diameters smaller than 30 nanometers (nm) - a single void is formed inside the shell during oxidation. This results in an asymmetric core of nickel, with a single void growing on one side of the core. The remaining core shrinks as the oxidation process continues. This is significant, in part, because the nickel oxide shell becomes progressively thicker on the side that abuts the core. The larger the core - within the 30 nm limit - the thicker that side of the shell becomes. In other words, you end up with a nickel oxide shell that can be significantly thicker on one side than the other.
However, the researchers found that larger nickel nanoparticles do something completely different. The researchers tested nanoparticles with nickel cores that were 96 nm in diameter, and found that the oxidation process in these nanoparticles created multiple voids in the core - though the core itself remained completely surrounded by the nickel oxide shell. This process effectively resulted in the creation of bubbles throughout the core. The "skeletons" of those bubbles still remained, even after full oxidation, creating an essentially hollow shell that was still criss-crossed with some remnants of the nickel core.
"This tells us a lot about how to create nanoscale structures using the nanoscale Kirkendall Effect," Tracy says. "It's a building block for future research in the field."
The study, "Size-Dependent Nanoscale Kirkendall Effect During the Oxidation of Nickel Nanoparticles," is published in the journal ACS Nano. The research was funded by the National Science Foundation and NC State, and is co-authored by Tracy, NC State undergraduate Justin Railsback, NC State Ph.D. student Aaron Johnston-Peck and former NC State postdoctoral research associate Dr. Junwei Wang.
The Department of Materials Science and Engineering is part of NC State's College of Engineering.
Note to editors: The study abstract follows.
"Size-Dependent Nanoscale Kirkendall Effect During the Oxidation of Nickel Nanoparticles"
Authors: Justin. G. Railsback, Aaron C. Johnston-Peck, Junwei Wang, Joseph B. Tracy, North Carolina State University
Published: April 2, 2010, ACS Nano
Abstract: The transformation of Ni nanoparticles (NPs) of different sizes (average diameters of 9 nm, 26 nm, and 96 nm) during oxidation to hollow (single void) or porous (multiple voids) NiO through the nanoscale Kirkendall effect was observed by transmission electron microscopy. Samples treated for 1-4 hours at 200-500 °C show that the structures of the completely oxidized NPs do not depend on the temperature, but oxidation proceeds more quickly at elevated temperatures. For the Ni/NiO system, after formation of an initial NiO shell (of thickness ~3 nm), single or multiple voids nucleate on the inner surface of the NiO shell, and the voids grow until conversion to NiO is complete. Differences in the void formation and growth processes cause size-dependent nanostructural evolution: For 9 nm and 26 nm NPs, a single void forms beneath the NiO shell, and the void grows by moving across the NP while conversion to NiO occurs opposite the site where the void initially formed. Due to differences in the Ni/NiO volume ratios for the 9 nm and 26 nm NPs when the void first forms, they have distinct nanostructures: The 9 nm NPs form NiO shells that are nearly radially symmetric, while there is a pronounced asymmetry in the NiO shells for 26 nm NPs. By choosing an intermediate oxidation temperature and varying the reaction time, partially oxidized Ni(core)/NiO(shell) NPs can be synthesized with good control. For 96 nm NPs, multiple voids form and grow, which results in porous NiO NPs.
####
About North Carolina State University
With more than 31,000 students and nearly 8,000 faculty and staff, North Carolina State University is a comprehensive university known for its leadership in education and research, and globally recognized for its science, technology, engineering and mathematics leadership.
NC State students, faculty and staff are focused. As one of the leading land-grant institutions in the nation, NC State is committed to playing an active and vital role in improving the quality of life for the citizens of North Carolina, the nation and the world.
For more information, please click here
Contacts:
Matt Shipman
News Services
919.515.6386
Dr. Joe Tracy
919.513.2623
Copyright © North Carolina State University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Nanoelectronics
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() Interdisciplinary: Rice team tackles the future of semiconductors Multiferroics could be the key to ultralow-energy computing October 6th, 2023
Interdisciplinary: Rice team tackles the future of semiconductors Multiferroics could be the key to ultralow-energy computing October 6th, 2023
![]() Key element for a scalable quantum computer: Physicists from Forschungszentrum Jülich and RWTH Aachen University demonstrate electron transport on a quantum chip September 23rd, 2022
Key element for a scalable quantum computer: Physicists from Forschungszentrum Jülich and RWTH Aachen University demonstrate electron transport on a quantum chip September 23rd, 2022
![]() Reduced power consumption in semiconductor devices September 23rd, 2022
Reduced power consumption in semiconductor devices September 23rd, 2022
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








