Home > Press > A novel Li-ion superconductor makes possible an era of safe battery: A fast Li-ion conducting solid electrolyte material comparable to the liquid electrolytes used in typical batteries. An innovative synthesis developed for a Li-ion superconductor without any compromise between p
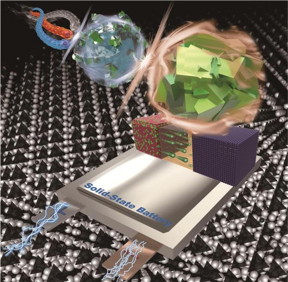 |
| Li-ion batteries based on liquid electrolytes are mainly used for batteries for electric vehicles and energy storage devices. However, as battery safety issues have recently been raised several times, various concerns about the use of existing batteries using flammable liquid electrolytes have increased. To solve this safety issue, all-solid-state battery technology, in which all battery components are replaced with solid materials, has recently attracted great attention. Dr. Kim's research team at KIST has developed a solid electrolyte with superionic conductivity using a sulfide-based crystalline structure called argyrodite. CREDIT Korea Institute of Science and Technology (KIST) |
Abstract:
Dr. Hyoungchul Kim's research team, from the Center for Energy Materials Research at the Korea Institute of Science and Technology (KIST, Acting President Yoon, Seok-jin), have successfully developed a sulfide-based *superionic conductor that can be used to a high-performance solid electrolyte in all-solid-state batteries. This new material delivers the Li-ion conductivity of 10.2 mS/cm at room temperature and is comparable to that of liquid electrolytes used for typical Li-ion batteries. The research team has also reported a new synthesis technology that can reduce the processing time of existing synthesis technologies by more than one third. We expect that this technology will greatly accelerate the mass production of superionic solid electrolyte materials and contribute to the commercialization of all-solid-state batteries.
A novel Li-ion superconductor makes possible an era of safe battery: A fast Li-ion conducting solid electrolyte material comparable to the liquid electrolytes used in typical batteries. An innovative synthesis developed for a Li-ion superconductor without any compromise between p
Sejong, Korea | Posted on April 10th, 2020* Superionic conductor: A material with high ion transport property corresponding to ion conductivity of 10 to 100 mS/cm at room temperature. These materials have been receiving much attention recently as they are used as electrolytes in various electrochemical devices such as batteries, fuel cells, and sensors.
Currently, Li-ion batteries based on liquid electrolytes are mainly used for batteries for electric vehicles and energy storage devices. However, as battery safety issues have recently been raised several times, various concerns about the use of existing batteries using flammable liquid electrolytes have increased. To solve this safety issue, all-solid-state battery technology, in which all battery components are replaced with solid materials, has recently attracted great attention. However, unlike a liquid electrolyte in which Li-ions can freely move, a solid electrolyte has a low Li-ion conductivity of 1/10 to 1/100 of that of a liquid electrolyte because the movement of Li-ions is confined within a rigid solid lattice. This is one of the most important and difficult challenges in the development of all-solid-state battery technology, and its technical and economic value is very great.
Dr. Kim's research team at KIST has developed a solid electrolyte with superionic conductivity using a sulfide-based crystalline structure called **argyrodite. Meanwhile, this crystal structure had high expectations for utilization due to its high Li-ion concentration and structural stability, but its Li-ion conductivity remained below 4 mS/cm due to the structural uniqueness of Li-ions trapped in the octahedral cage in the argyrodite crystal. The research team has newly developed a novel Li-ion pathway that crosses the octahedral cage by applying a technique for selectively substituting chlorine, a halogen element, at specific atomic positions. The new solid electrolyte material, developed by KIST researchers, has an Li-ion conductivity of 10.2 mS/cm, which is equivalent to that of a conventional liquid electrolyte at room temperature, and still maintains electrochemical stability under various battery operating conditions.
**Argyrodite: A crystal structure that was discovered in the mineral of Ag8GeS6 by Clemens Winkler in 1886. Recently, much research has been conducted on replacing cation sites with alkali metals such as Li and applying them to electrochemical devices.
In addition, the new synthesis method reported by KIST research team has attracted more attention as it is possible to maximize the mass productivity of superionic solid electrolyte materials. While the conventional ***solid-state reaction process requires more than several days of processing time, this study proposed a simple synthesis method that combines the nanocrystalline nucleation process and an infrared rapid heat treatment technology to shorten the process time to within 10 hours.
***Solid-state reaction process: A dry-based material process in which one or more solid reaction products are formed with the diffusion of elementary particles. It generally requires high-temperature heat treatment in order to make reactions to occur at an appropriate rate.
According to Dr. Kim, "In the field of all-solid-state battery technology, foreign researchers including Japan are leading the research. In this study, there is great significance in developing a high-performance solid electrolyte material with excellent mass productivity." He further comments, "Synthesis of superionic sulfide materials through rapid process is very likely to be commercialized, and can be widely used in electric vehicles and energy storage system as a solid electrolyte in the future."
###
The research was supported by the Dual Use Technology Program of the Institute of Civil Military Technology Cooperation granted financially resources from the Ministry of Trade, Industry and Energy (Ministrer Sung, Yun-mo) and the Defense Acquisition Program Administration (Minister Wang, Jung-Hong). This work was also supported by the Institutional Research Program of the Korea Institute of Science and Technology and the Technology Development Program to Solve Climate Changes of the National Research Foundation funded by the Ministry of Science and ICT (Minister Choi, Ki-young). The results of this study have been published online in Nano Letters (IF: 12.279, top 5.743% of JCR), a prestigious international journal in the areas of nanoscience and nanotechnology.
####
For more information, please click here
Contacts:
Kim, Do-Hyun
82-295-86344
Copyright © National Research Council of Science & Technology(NST)
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Superconductivity
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








