Home > Press > A rapid extension of nanographene sheets from readily available hydrocarbons: Precisely controlled synthesis of structurally uniform carbon sheets could advance nanographene materials science
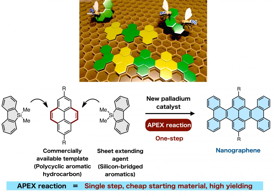 |
| Selective annulation at the 'K-region' leads to direction-controlled synthesis of nanographenes in high yield. CREDIT: ITbM, Nagoya University |
Abstract:
Commercially available hydrocarbons were used as templates to synthesize uniform nanographene sheets using a series of sheet extending agents and a new transition metal catalyst, which were developed by Kyohei Ozaki, Katsuaki Kawasumi, Mari Shibata, Hideto Ito, and Professor Kenichiro Itami at Nagoya University's Institute of Transformative Bio-Molecules (ITbM) and of the JST-ERATO Itami Molecular Nanocarbon Project.
A rapid extension of nanographene sheets from readily available hydrocarbons: Precisely controlled synthesis of structurally uniform carbon sheets could advance nanographene materials science
Nagoya, Japan | Posted on February 17th, 2015Nanographenes, which are nanometer-sized graphenes, possess good semiconducting properties making them highly promising materials for the next generation of organic electronic devices. Itami and his team have developed a new catalytic one-shot annulative π (pi)-extension (APEX) reaction, which enables the rapid construction of nanographene in a single-step. The study, published online on February 16, 2015 in Nature Communications, illustrates the discovery of efficient sheet extending agents and a highly reactive palladium catalyst, making the APEX reaction highly applicable towards the 'growth from template' construction of a variety of nanographene structures as well as fine-tuning of their properties.
The physical properties of nanographenes are mainly determined by their width, length and edge structures. Therefore, structural control on a nanometer-scale is highly desirable to access useful nanographenes. Many nanographene syntheses reported up to now involve a two-step sequence of (i) assembly of small aromatic components, followed by (ii) stitching (graphenization) of polycyclic precursors to make them into sheets. Although these methodologies have been useful, multiple steps are required for the assembly of molecules. In addition, incomplete stitching, side reactions and low yields have caused difficulty in the precise control for the synthesis of nanographenes. To overcome this, Itami and his co-workers have proposed a single-step APEX reaction, which involves selective attachment and annulation of an aromatic π-extending agent on a specific region of readily available polycyclic aromatic hydrocarbons (PAHs).
Up until now, there have been no reported examples of APEX reactions conducted at the convex armchair edge of PAHs, otherwise known as the K region. Reactions at the K-region are considered to be difficult as other C-H positions are usually more prone to aromatic substitution reactions.
"We started to work on developing APEX reactions when we found a palladium catalyst that led to selective arylation (attachment of an aromatic group) at the K-region," says Itami, the Director of ITbM and the JST-ERATO project. "After stitching, we were able to synthesize warped nanographenes in two-steps. Based on this insight, we envisaged that we could selectively activate the K-region and induce cyclization in one-step by careful choice of catalysts and reactants."
"As far as we know, there have been no examples on the use of PAHs as starting materials for the synthesis of nanographenes," says Ito, a co-author of this study. "Although PAHs are relatively cheap and readily available, their relatively low reactivity and difficulty in selectively installing functionalities have limited their use as substrates," he adds. "Therefore, we carried out extensive screening of palladium salts, additives, and sheet (π)-extending agents to find the right combination of reagents that induced the APEX reaction on PAHs to generate uniform sheets of nanographene."
"The most surprising part of this research was that a silicon-bridged aromatic compound could be used as a sheet extending agent for the APEX reaction," says Ozaki and Kawasumi, graduate students of Nagoya University who conducted the experiments. "The silicon-bridged molecule was a product of a reaction, in which we were actually expecting a different product. Because we had the molecule in hand, we decided to use it in the catalytic system and coincidently found that selective annulation at the K-region had occurred."
In order to investigate the nature of this APEX reaction, computational studies using density functional theory calculations were conducted on a model reaction. "We predict that the selectivity of the APEX reaction arises from the K-region π-bond having the least aromatic character compared to other sites," says Shibata, who performed the theoretical calculations. "Further computational studies will be carried out to find more details on what is actually happening in the APEX reaction."
Functional handles, such as chloride and boryl groups on the substrates were compatible under the APEX reaction conditions, which makes the products ready for further functionalization. The synthetic utility of the APEX reaction was also demonstrated by examining multiple and sequential APEX reactions to successfully access larger nanographene frameworks on a multi-gram scale. "The most difficult part of this research was by far the solubility issue that we had with handling the nanographene products," says Ozaki and Ito. "Most of them were insoluble in the solvents that we tried. However, I was sure that I could make this reaction better and I believe that it was my determination to accomplish the precisely controlled synthesis of nanographenes that finally led to this outcome," continues Ozaki.
"Through the development of the APEX reaction, we have succeeded in the direction-controlled growth of nanographene sheets from PAHs," says Itami. "We believe that this methodology will lead to the synthesis of uniform nanographenes that are essential for future nanomaterial devices, and will also be applicable for constructing other exciting conjugated structures in a rapid and programmable manner."
####
About ITbM, Nagoya University
The World Premier International Research Center Initiative (WPI) for the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. As part of the Japanese science ministry's MEXT program, ITbM aims to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a "Mix-Lab" style, where international young researchers from multidisciplinary fields work together side-by-side in the same lab. Through these endeavors, ITbM will create "transformative bio-molecules" that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
About JST-ERATO Itami Molecular Nanocarbon Project
This project entails the design and synthesis of as-yet largely unexplored nanocarbons as structurally well-defined molecules, and the development of novel, highly functional materials based on these nanocarbons. Through the combination of chemical and physical methods, the project aims to achieve the controlled synthesis of well-defined uniquely structured nanocarbon materials. Interdisciplinary research is conducted to encompass the control of molecular arrangement and orientation, structural and functional analysis, and applications in devices and biology.
About JST-ERATO
ERATO (The Exploratory Research for Advanced Technology), one of the Strategic Basic Research Programs, aims to form a headstream of science and technology, and ultimately contribute to science, technology, and innovation that will change society and the economy in the future. In ERATO, a Research Director, a principal investigator of ERATO research project, establishes a new research base in Japan and recruits young researchers to implement his or her challenging research project within a limited time frame.
For more information, please click here
Contacts:
Author Contact
Professor Kenichiro Itami
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-788-6098
About ERATO Program
Takeshi Ohyama
Department of Research Project, Japan Science and Technology Agency (JST)
K's Goban-cho, 7 Goban-cho, Chiyoda-ku Tokyo 102-0076, Japan
TEL: +81-3-3512-3528 FAX: +81-3-3222-2068
Media Contact
Dr. Ayako Miyazaki
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-4999 FAX: +81-52-789-3240
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
Copyright © ITbM, Nagoya University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Graphene/ Graphite
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
![]() Breakthrough in proton barrier films using pore-free graphene oxide: Kumamoto University researchers achieve new milestone in advanced coating technologies September 13th, 2024
Breakthrough in proton barrier films using pore-free graphene oxide: Kumamoto University researchers achieve new milestone in advanced coating technologies September 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








