Home > Press > Research and applications of iron oxide nanoparticles
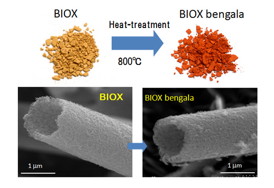 |
| Fig.1 Novel red-colored iron oxide |
Abstract:
From the mysteries of producing red colors in traditional Japanese Bizen stoneware to iron-oxidizing bacteria for lithium ion batteries, Professor Jun Takada is at the forefront of research on innovative iron oxide nanomaterials. This research is featured in the December issue of the Okayama University eBulletin: www.okayama-u.ac.jp/user/kouhou/ebulletin/feature/vol6/feature_001.html
Research and applications of iron oxide nanoparticles
Okayama, Japan | Posted on February 26th, 2014Professor Jun Takada is at the Graduate School of Natural Science and Technology at Okayama University. "I spent thirty years investigating how craftsman were able to render the beautiful red colors in Bizen and Arita pottery," explains Takada. "This research revealed the important role of iron oxide particles for producing the colors. I am now working on innovative applications of nanometer scale iron oxide materials produced by 'iron-oxidizing bacteria'. I have made a transition from fine ceramics and Bizen stoneware to fuel cells and biotechnology!"
Bizen ware has a history of more than a thousand years. The pottery has distinctive 'hidasuki' or 'fire-marked' reddish-brown colors(Fig.1) and is produced using iron rich clay mined from rice fields in the Bizen area of Okayama Prefecture. Intriguingly, the red colors are rendered by wrapping straw around the stoneware and not by glazing. But why does the straw, which was originally used to separate pieces of stoneware in kilns, produce the red colors where the straw is in contact with the surface of the clay?
"Our research showed the Bizen clay had a high content of iron lesser concentrations of other elements including silicon, calcium, magnesium, and sodium," explains Takada. "The red patterns are produced by the precipitation of corundum (α-Al2O3) followed by the formation of hermatite (α-Fe2O3) around it during the cooling process."
More specifically, potassium in the straw reduces the melting point of the surface of the Bizen clay, which leads to the formation of an approximately 50 micrometer thick liquid in the surface of the hot clay, where the aforementioned reactions occur. Furthermore, the research identified the formation of sandwich like crystals of α-Fe2O3/α-Al2O3/α-Fe2O3 particles during the reaction in the slow cooling.
"The main outcome of the research was the importance of hematite in formation of the hidasuki-red patterns," says Takada. "We also found a relationship between the growth of hematite particles and the color of the resulting Bizen ware."
Takada and colleagues also produced so called Al-substituted hematite, where the substitution of Al suppressed grain growth of hematite and the tone color became stronger with increasing aluminum. They found that particles of about 100 nm produced yellowish red, and larger particles sizes led to red and eventually dark purple colors. This research finally enabled the researchers to produce hematite based powders that do not contain hazardous elements such as chrome or lead, and there by increases the range of applications of these materials, especially producing Aka-e decoration on the over glazed Arita ware.
Inspired by his research on hematite and iron oxide particles for producing red colors, Takada initiated new research on the preparation of nanostructure tubes and fibers of iron oxides—known as biogenous iron oxides (BIOX)(Fig.1)—produced by so-called iron-oxidizing bacteria. "The yellowish brown precipitate found in a groundwater spring is due to the presence of extracellular fibrous bundles produced by iron oxidizing bacteria such as Leptothrix ochracea," says Takada. "Our research shows that this otherwise useless looking material has some extremely important applications." Indeed, research by Takada on the physical properties of the BIOX matrix showed this iron oxide to have an amorphous state made of organic/inorganic hybrid structure of ~3 nm sized nanoparticles of a many different elements including carbon, phosphorous, silicon, and iron.
Important applications of BIOX include as an anode material of Li-ion batteries, catalysts, color pigmentation, and innovation based on this materials high affinity to human cells. "Our studies on the formation of BIOX show that extracellular secretion of bacterial polymers triggers deposition and binding of aquatic inorganics such as Fe, Si, and P, which results in the unique organic/inorganic hybrid," says Takada. "This low cost BIOX is an eco-friendly and nontoxic functional material with a wide range of applications, including producing fine ceramics and arts, which are the roots of this research."
Publications
T. Ema, et al, "Robust porphyrin catalysts immobilized on biogenous iron oxide for the repetitive conversions of epoxides and CO2 into cyclic carbonates", Green Chemistry, 15 , 2485, (2013)
H. Hashimoto, et al, "Nano-micrometer-architectural acidic silica preparaed from iron oxide of Leptothrix ochracea origin", Applied Materials & Interfaces, 5, 5194, (2013).
H. Ishihara, et al, "Initial parallel arrangement of extracellular fibrils holds a key for sheath frame construction by Leptothrix sp. strain OUMS1", Minerals, 3, 73, (2013).
H. Hashimoto, et al, "Preparation, microstructue, and color tone of microtubule material composed of hematite/amorphous-silicate nonocomposite from iron oxide of bacterial origin", Dyes and Pigments, 95, 639, (2012).
J. Takada and H. Hashimoto, "Characteristics of biogenous iron oxide microtubes formed by iron-oxidizing bacteria, Leptothrix ochracea" , Handbook of Metal Biotechnology, ed. by M. Ike et al, Pan Stanford Publishing, pp.139, (2012).
T. Suzuki et al, "Environmental microbiology: silicon and phosphorus linkage with iron via oxygen in the amorphous matrix of Gallionella ferruginea stalks", Applied and Environmental Microbiology, 78, 236 (2012).
K. Mandai et al, "Iron oxide-immobilized palladium catalyst for the solvent-free Suzuki-Miyaura coupling reaction", Tetrahedron Letters, 53, 329, (2012).
T. Ema et al, "Highly active lipase immobilized on biogenous iron oxide via an organic bridging group: the dramatic effect of the immobilization support on enzymatic function," Green Chemistry, 13, 3187 (2011).
M. Furutani et al, "Initial assemblage of bacterial saccharic fibrils and element deposition to form an immature sheath in cultured Leptothrix sp. strain OUMS1", Minerals, 1, 157, (2011).
T. Sakai et al, "Chemical modification of biogenous iron oxide to create an excellent enzyme scaffold," Organic Biomolecular Chemistry, 8, 336 (2010).
Y. Kusano, et al, "Science in the art of the master Bizen potter", Accounts of Chemical Research, 43, 906, (2010).
H. Asaoka, et al, "Reproduction of Japanese traditional pigment based on iron oxide powders with yellowish red color", Materials Research Society Symposium Proceedings, 712, 435, (2002).
####
About Okayama University
Okayama University is one of the largest comprehensive universities in Japan with roots going back to the Medical Training Place sponsored by the Lord of Okayama and established in 1870. Now with 1,300 faculty and 14,000 students, the University offers courses in specialties ranging from medicine and pharmacy to humanities and physical sciences. Okayama University is located in the heart of Japan approximately 3 hours west of Tokyo by Shinkansen.
For more information, please click here
Contacts:
Okayama University
1-1-1 Tsushima-naka , Kita-ku ,
Okayama 700-8530, Japan
Planning and Public Information Division
Copyright © Okayama University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








