Home > Press > Small and efficient - water nanodroplets cool biomolecules ultrafast
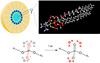 |
| Upper left: Schematic of a reverse micelle consisting of phospholipid molecules. The phosphate groups of the lipid molecules (blue spheres) are arranged at the inner surface of the micelle. Water molecules are located in the inner part of the micelle. Upper right: Enlarged view of the structure of a phospholipid molecule. Oxygen atoms are shown in red, hydrogen atoms in white, carbon atoms in grey, the nitrogen atom in blue, and the phosphorus atom in orange. The angled water molecules are arranged around the phosphate (PO4) group. Lower part: Scheme of energy transfer. In the experiments, the (asymmetric) phosphate vibration is initially excited (red oxygen atoms). The energy released in the decay of the vibration is transferred to the surrounding water shell (red H2O molecules) within 1 ps. |
Abstract:
Researchers of the Max-Born-Institute at Berlin, Germany, have observed how biomolecules transfer energy into extremely small water droplets in their environment. A water shell consisting of only 3 water molecules around a phospholipid molecule is sufficient for energy transfer within 1 ps.
Small and efficient - water nanodroplets cool biomolecules ultrafast
Berlin, Germany | Posted on December 2nd, 2012Biochemical processes occur mainly in an aqueous environment. Particular groups of a biomolecule are embedded in a shell of water molecules, a process called hydration. The water shell stabilizes the biomolecular structure and enables an exchange of energy between the biomolecule and its environment. Examples are the double helix of DNA, the carrier of basic genetic information, in an aqueous medium and the membranes of living cells which consist of phospholipids. The molecular mechanisms, the speed and the efficiency of energy exchange between the biomolecule and the water shell are understood only in part and, thus, a topic of current basic research.
Scientists of the Max-Born-Institute have shown that extremely small water droplets embedding a phospholipid molecule enable efficient energy transfer on a time scale of 1 ps (1 ps = 10-12 s = 1 millionth of a millionth of a second). René Costard, Christian Greve, Ismael Heisler, and Thomas Elsaesser report in the current issue of Journal of Physical Chemistry Letters (vol.3, page 3646, 2012) that 3 water molecules around the phosphate group of the phospholipid are sufficient for transferring the energy of vibrations from the phospholipid into this minimal water shell. The transferred energy heats the water shell by 10 to 20 centigrades. The thermal energy is stored in tilting motions of water molecules, so called librations, and leads to a weakening of the interaction between the water molecules, the so called hydrogen bonds. The overall molecular structure of the water shell remains practically unchanged. This extremely efficient mechanism of energy disposal allows for the transfer of even larger amounts of energy, protecting the biomolecule against damage by overheating.
The researchers studied a phospholipid model system consisting of the DOPC molecules shown in Fig. 1. The molecules are arranged in so-called reverse micelles which contain the water molecules hydrating the phosphate groups. In this geometry, the hydration level, i.e., water content, can be changed in a wide range. For studying energy transfer, either phosphate vibrations of the phospholipid or OH stretching vibrations of water are excited by an infrared pulse of a 0.1 ps duration. The vibrations decay within a fraction of a picosecond and the energy released in this decay is transferred into the water shell. The transfer and redistribution of energy is mapped via transient two-dimensional infrared spectra of the OH stretching vibration of water. The weakening of hydrogen bonds in the heated water shell leads to a shift of the OH stretching spectra to higher frequencies. Measuring the change of the two-dimensional spectra as a function of time provides direct insight into the energy transfer dynamics.
Full bibliographic informationR. Costard, C. Greve, I. A. Heisler, T. Elsaesser: Ultrafast energy redistribution in local hydration shells of phospholipids: a two-dimensional infrared study. J. Phys. Chem. Lett. 3, 3646 (2012).
####
For more information, please click here
Contacts:
Thomas Elsaesser
Copyright © AlphaGalileo
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Physics
![]() Quantum computers simulate fundamental physics: shedding light on the building blocks of nature June 6th, 2025
Quantum computers simulate fundamental physics: shedding light on the building blocks of nature June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Magnetism in new exotic material opens the way for robust quantum computers June 4th, 2025
Magnetism in new exotic material opens the way for robust quantum computers June 4th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








