Home > Press > Silicon-carbon electrodes snap, swell, don't pop: Nanocomposite electrodes being charged with electricity reveal performance advantages that could lead to longer-lasting, cheaper vehicle batteries
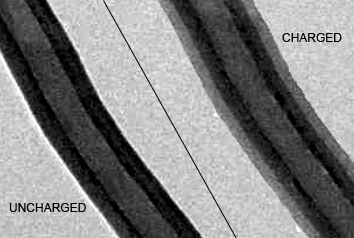 |
| This composite image shows a silicon-carbon nanofiber electrode before (left) and after (right) being charged with lithium ions. |
Abstract:
A study that examines a new type of silicon-carbon nanocomposite electrode reveals details of how they function and how repeated use could wear them down. The study also provides clues to why this material performs better than silicon alone. With an electrical capacity five times higher than conventional lithium battery electrodes, silicon-carbon nanocomposite electrodes could lead to longer-lasting, cheaper rechargeable batteries for electric vehicles.
Silicon-carbon electrodes snap, swell, don't pop: Nanocomposite electrodes being charged with electricity reveal performance advantages that could lead to longer-lasting, cheaper vehicle batteries
Richland, WA | Posted on March 14th, 2012Published online in the journal Nano Letters last week, the study includes videos of the electrodes being charged at nanometer-scale resolution. Watching them in use can help researchers understand the strengths and weaknesses of the material.
"The electrodes expand as they get charged, and that shortens the lifespan of the battery," said lead researcher Chongmin Wang at the Department of Energy's Pacific Northwest National Laboratory. "We want to learn how to improve their lifespan, because silicon-carbon nanofiber electrodes have great potential for rechargeable batteries."
Plus & Minus
Silicon has both advantages and disadvantages for use as a battery material. It has a high capacity for energy storage, so it can take on a hefty charge. Silicon's problem, though, is that it swells up when charged, expanding up to 3 times its discharged size. If silicon electrodes are packed tightly into a battery, this expansion can cause the batteries to burst. Some researchers are exploring nano-sized electrodes that perform better in such tight confines.
A multi-institution group led by PNNL's Wang decided to test nano-sized electrodes consisting of carbon nanofibers coated with silicon. The carbon's high conductivity, which lets electricity flow, nicely complements silicon's high capacity, which stores it.
Researchers at DOE's Oak Ridge National Laboratory in Oak Ridge, Tenn., Applied Sciences Inc. in Cedarville, Ohio, and General Motors Global R&D Center in Warren, Mich. created carbon nanofibers with a thin layer of silicon wrapped around. They provided the electrodes to the team at PNNL to probe their behavior while functioning.
First, Wang and colleagues tested how much lithium the electrodes could hold and how long they lasted by putting them in a small testing battery called a half-cell. After 100 charge-discharge cycles, the electrodes still maintained a very good capacity of about 1000 milliAmp-hours per gram of material, five to 10 times the capacity of conventional electrodes in lithium ion batteries.
Although they performed well, the team suspected that the expansion and contraction of the silicon could be a problem for the battery's longevity, since stretching tends to wear things out. To determine how well the electrodes weather the repeated stretching, Wang popped a specially designed, tiny battery into a transmission electron microscope, which can view objects nanometers wide, in DOE's EMSL, the Environmental Molecular Sciences Laboratory on the PNNL campus.
They zoomed in on the tiny battery's electrode using a new microscrope that was funded by the Recovery Act. This microscope allowed the team to study the electrode in use, and they took images and video while the tiny battery was being charged and discharged.
Not Crystal Glass
Previous work has shown that charging causes lithium ions to flow into the silicon. In this study, the lithium ions flowed into the silicon layer along the length of the carbon nanofiber at a rate of about 130 nanometers per second. This is about 60 times faster than silicon alone, suggesting that the underlying carbon improves silicon's charging speed.
As expected, the silicon layer swelled up about 300 percent as the lithium entered. However, the combination of the carbon support and the silicon's unstructured quality allowed it to swell evenly. This compares favorably to silicon alone, which swells unevenly, causing imperfections.
In addition to swelling, lithium is known to cause other changes to the silicon. The combination of lithium and silicon initially form an unstructured, glassy layer. Then, when the lithium to silicon ratio hits 15 to 4, the glassy layer quickly crystallizes, as previous work by other researchers has shown.
Wang and colleagues examined the crystallization process in the microscope to better understand it. In the microscope video, they could see the crystallization advance as the lithium filled in the silicon and reached the 15 to 4 ratio.
They found that this crystallization is different from the classic way that many substances crystallize, which builds from a starting point. Rather, the lithium and silicon layer snapped into a crystal all at once when the ratio hit precisely 15 to 4. Computational analyses of this crystallization verified its snappy nature, a type of crystallization known as congruent phase transition.
But the crystallization wasn't permanent. Upon discharging, the team found that the crystal layer became glassy again, as the concentration of lithium dropped on its way out of the silicon.
To determine if repeated use left its mark on the electrode, the team charged and discharged the tiny battery 4 times. Comparing the same region of the electrode between the first and fourth charging, the team saw the surface become rough, similar to a road with potholes.
The surface changes were likely due to lithium ions leaving a bit of damage in their wake upon discharging, said Wang. "We can see the electrode's surface go from smooth to rough as we charge and discharge it. We think as it cycles, small defects occur, and the defects accumulate."
But the fact that the silicon layer is very thin makes it more durable than thicker silicon. In thick silicon, the holes that lithium ions leave behind can come together to form large cavities. "In the current design, because the silicon is so thin, you don't get bigger cavities, just like little gas bubbles in shallow water come up to the surface. If the water is deep, the bubbles come together and form bigger bubbles."
In future work, researchers hope to explore the thickness of the silicon layer and how well it bonds with the underlying carbon to optimize the performance and lifetime of the electrodes.
####
About DOE/Pacific Northwest National Laboratory
EMSL, the Environmental Molecular Sciences Laboratory, is a national scientific user facility sponsored by the Department of Energy's Office of Science. Located at Pacific Northwest National Laboratory in Richland, Wash., EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. Its integrated computational and experimental resources enable researchers to realize important scientific insights and create new technologies.
Interdisciplinary teams at Pacific Northwest National Laboratory address many of America's most pressing issues in energy, the environment and national security through advances in basic and applied science. PNNL employs 4,700 staff, has an annual budget of nearly $1.1 billion, and has been managed for the U.S. Department of Energy by Ohio-based Battelle since the laboratory's inception in 1965.
For more information, please click here
Contacts:
Mary Beckman
509-375-3688
Copyright © DOE/Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








