Home > Press > Studying Argon Gas Trapped in Two-Dimensional Array of Tiny "Cages": Understanding how individual atoms enter and exit the nanoporous frameworks could help scientists design new materials for gas separation and nuclear waste remediation
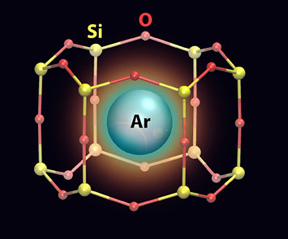 |
| An artistic rendering of an argon (Ar) atom trapped in a nanocage that has a silicon (Si)-oxygen (O) framework. |
Abstract:
Scientists at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory had just finished an experiment with a two-dimensional (2D) structure they synthesized for catalysis research when, to their surprise, they discovered that atoms of argon gas had gotten trapped inside the structure's nanosized pores. Argon and other noble gases have previously been trapped in three-dimensional (3D) porous materials, but immobilizing them on surfaces had only been achieved by either cooling the gases to very low temperatures to condense them, or by accelerating gas ions to implant them directly into materials.
Studying Argon Gas Trapped in Two-Dimensional Array of Tiny "Cages": Understanding how individual atoms enter and exit the nanoporous frameworks could help scientists design new materials for gas separation and nuclear waste remediation
Upton, NY | Posted on July 17th, 2017"We are the first team to trap a noble gas in a 2D porous structure at room temperature," said Anibal Boscoboinik, a materials scientist at Brookhaven Lab's Center for Functional Nanomaterials [https://www.bnl.gov/cfn/] (CFN), a DOE Office of Science User Facility where part of the research was conducted.
This achievement, reported in a paper published today in Nature Communications [ https://www.nature.com/articles/ncomms16118 ], will enable scientists to use traditional surface-science tools-such as x-ray photoelectron and infrared reflection absorption spectroscopy-to perform detailed studies of single gas atoms in confinement. The knowledge gained from such research could inform the design, selection, and improvement of adsorbent materials and membranes for capturing gases such as radioactive krypton and xenon generated by nuclear power plants.
The team of scientists from Brookhaven Lab, Stony Brook University, and the National University of San Luis in Argentina synthesized 2D aluminosilicate (composed of aluminum, silicon, and oxygen) films on top of a ruthenium metal surface. The scientists created this 2D model catalyst material to study the chemical processes happening in the industrially used 3D catalyst (called a zeolite), which has a cage-like structure with open pores and channels the size of small molecules. Because the catalytically active surface is enclosed within these cavities, it is difficult to probe with traditional surface-science tools. The 2D analogue material has the same chemical composition and active site as the 3D porous zeolite but its active site is exposed on a flat surface, which is easier to access with such tools.
To confirm that the argon atoms were trapped in these "nanocages," the scientists exposed the 2D material to argon gas and measured the kinetic energy and number of electrons ejected from the surface after striking it with an x-ray beam. They performed these studies at the former National Synchrotron Light Source I (NSLS-I) and its successor facility, NSLS-II [ https://www.bnl.gov/ps/ ] (both DOE Office of Science User Facilities at Brookhaven), with an instrument developed and operated by the CFN. Because the binding energies of core electrons are unique to each chemical element, the resulting spectra reveal the presence and concentration of elements on the surface. In a separate experiment conducted at the CFN, they grazed a beam of infrared light over the surface while introducing argon gas. When atoms absorb light of a specific wavelength, they undergo changes in their vibrational motions that are specific to that element's molecular structure and chemical bonds.
To get a better understanding of how the framework itself contributes to caging, the scientists investigated the trapping mechanism with silicate films, which are similar in structure to the aluminosilicates but contain no aluminum. In this case, they discovered that not all of the argon gets trapped in the cages-a small amount goes to the interface between the framework and ruthenium surface. This interface is too compressed in the aluminosilicate films for argon to squeeze in.
After studying adsorption, the scientists examined the reverse process of desorption by incrementally increasing the temperature until the argon atoms completely released from the surface at 350 degrees Fahrenheit. They corroborated their experimental spectra with theoretical calculations of the amount of energy associated with argon entering and leaving the cages.
In another infrared spectroscopy experiment conducted in Brookhaven's Chemistry Division [ https://www.bnl.gov/chemistry/ ], they explored how the presence of argon in the cages affects the passage of carbon monoxide molecules through the framework. They found that argon restricts the number of molecules that adsorb onto the ruthenium surface.
"In addition to trapping small atoms, the cages could be used as molecular sieves for filtering carbon monoxide and other small molecules, such as hydrogen and oxygen," said first author Jian-Qiang Zhong, a CFN research associate.
While their main goal going forward will be to continue investigating zeolite catalytic processes on the 2D material, the scientists are interested in learning the impact of different pore sizes on the materials' ability to trap and filter gas molecules.
"As we seek to better understand the material, interesting and unexpected findings keep coming up," said Boscoboinik. "The ability to use surface-science methods to understand how a single atom of gas behaves when it is confined in a very small space opens up lots of interesting questions for researchers to answer."
This research used resources of the National Energy Research Scientific Computing Center [ http://www.nersc.gov ], a DOE Office of Science User Facility at Lawrence Berkeley National Laboratory, and was supported by Brookhaven's Laboratory Directed Research and Development
[https://science.energy.gov/lp/laboratory-directed-research-and-development/ ] program and the National Scientific and Technical Research Council (CONICET) of Argentina.
####
About Brookhaven National Laboratory
Brookhaven National Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov [ http://science.energy.gov/ ].
For more information, please click here
Contacts:
Ariana Tantillo
(631) 344-2347
or
Peter Genzer
(631) 344-3174
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
Thin films
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() New study introduces the best graphite films: The work by Distinguished Professor Feng Ding at UNIST has been published in the October 2022 issue of Nature Nanotechnology November 4th, 2022
New study introduces the best graphite films: The work by Distinguished Professor Feng Ding at UNIST has been published in the October 2022 issue of Nature Nanotechnology November 4th, 2022
![]() Thin-film, high-frequency antenna array offers new flexibility for wireless communications November 5th, 2021
Thin-film, high-frequency antenna array offers new flexibility for wireless communications November 5th, 2021
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
2 Dimensional Materials
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Zeolites
![]() Dendritic fibrous nanosilica: all-in-one nanomaterial for energy, environment and health November 4th, 2017
Dendritic fibrous nanosilica: all-in-one nanomaterial for energy, environment and health November 4th, 2017
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Research partnerships
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








