Home > Press > Researchers peer into atom-sized tunnels in hunt for better battery: May improve lithium ion for larger devices, like cars
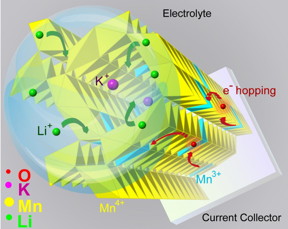 |
| 3-D schematic shows how doping with potassium may facilitate the insertion of lithium ions into manganese dioxide coated on a current collector. CREDIT Reza Shahbazian-Yassar/UIC |
Abstract:
Battery researchers seeking improved electrode materials have focused on "tunneled" structures that make it easier for charge-carrying ions to move in and out of the electrode. Now a team led by a researcher at the University of Illinois at Chicago has used a special electron microscope with atomic-level resolution to show that certain large ions can hold the tunnels open so that the charge-carrying ions can enter and exit the electrode easily and quickly.
Researchers peer into atom-sized tunnels in hunt for better battery: May improve lithium ion for larger devices, like cars
Chicago, IL | Posted on December 8th, 2016The finding is reported in Nature Communications.
"Significant research has been done to increase the energy density and power density of lithium ion battery systems," says Reza Shahbazian-Yassar, associate professor of mechanical and industrial engineering at UIC.
The current generation, he said, is useful enough for portable devices, but the maximum energy and power that can be extracted is limiting.
"So for an electric car, we need to increase the energy and power of the battery -- and decrease the cost as well," he said.
His team, which includes coworkers at Argonne National Laboratory, Michigan Technological University and the University of Bath in the U.K., has focused on developing a cathode based on manganese dioxide, a very low cost and environmentally-friendly material with high storage capacity.
Manganese dioxide has a lattice structure with regularly spaced tunnels that allow charge carriers -- like lithium ions -- to move in and out freely.
"But for the tunnels to survive for long-lasting function, they need support structures at the atomic scale," Shahbazian-Yassar said. "We call them tunnel stabilizers, and they are generally big, positive ions, like potassium or barium."
But the tunnel stabilizers, being positively charged like the lithium ions, should repel each other.
"If lithium goes in, will the tunnel stabilizer come out?" Shahbazian-Yassar shrugged. "The research community was in disagreement about the role of tunnel stabilizers during the transfer of lithium into tunnels. Does it help, or hurt?"
The new study represents the first use of electron microscopy to visualize the atomic structure of tunnels in a one-dimensional electrode material -- which the researchers say had not previously been possible due to the difficulty of preparing samples. It took them two years to establish the procedure to look for tunnels in potassium-doped nanowires of manganese dioxide down to the single-atom level.
Yifei Yuan, a postdoctoral researcher working jointly at Argonne National Laboratory and UIC and the lead author on the study, was then able to use a powerful technique called aberration-corrected scanning transmission electron microscopy to image the tunnels at sub-ångstrom resolution so he could clearly see inside them -- and he saw they do change in the presence of a stabilizer ion.
"It's a direct way to see the tunnels," Yuan said. "And we saw that when you add a tunnel stabilizer, the tunnels expand, their electronic structures also change, and such changes allow the lithium ions to move in and out, around the stabilizer."
The finding shows that tunnel stabilizers can help in the transfer of ions into tunnels and the rate of charge and discharge, Shahbazian-Yassar said. The presence of potassium ions in the tunnels improves the electronic conductivity of manganese dioxide and the ability of lithium ions to diffuse quickly in and out of the nanowires.
"With potassium ions staying in the center of the tunnels, the capacity retention improves by half under high cycling current, which means the battery can hold on to its capacity for a longer time," he said.
###
Co-authors on the Nature Communications paper are Kun He, Soroosh Sharifi-Asl, Boao Song and Anmin Nie of UIC; Chun Zhan, Zhenzhen Yang, Xiangyi Luo, Hao Wang, Khalil Amine and Jun Lu of Argonne; Hungru Chen, Stephen M. Wood and M. Saiful Islam of the University of Bath; and Wentao Yao of Michigan Tech.
Funding was provided by the National Science Foundation and the U.S. Department of Energy.
####
For more information, please click here
Contacts:
Bill Burton
312-996-2269
Copyright © University of Illinois at Chicago
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Research partnerships
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








