Home > Press > Nanobiotix Provides Update on Global Development of Lead Product NBTXR3: Seven clinical trials across the world: More than 2/3 of STS patients recruited in the “act.in.sarc” Phase II/III trial: Phase I/II prostate cancer trial now recruiting in the U.S.
 |
Abstract:
NANOBIOTIX (Euronext: NANO – ISIN: FR0011341205), a late clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer, today provides an update on the global development of its lead product, NBTXR3, across all indications.
Nanobiotix Provides Update on Global Development of Lead Product NBTXR3: Seven clinical trials across the world: More than 2/3 of STS patients recruited in the “act.in.sarc” Phase II/III trial: Phase I/II prostate cancer trial now recruiting in the U.S.
Paris, France and Cambridge, MA | Posted on November 28th, 2016NBTXR3 is a first-in-class radio-enhancer nanoparticle designed for direct injection into cancerous tumors. NBTXR3 has the potential to improve radiotherapy efficacy by destroying locally advanced tumors more efficiently. It has been engineered to increase the local absorption of the radiotherapy dose and thereby increasing the efficacy of radiotherapy with the benefit of not increasing toxicity or causing damage to surrounding healthy tissues.
Elsa BORGHI, CMO of Nanobiotix said: “We made significant progress this year with the global clinical development of NBTXR3 across seven clinical trials and we are looking forward to potentially obtain a CE mark in 2017. Our filing was based on the scale and significance of the results already seen. The clinical and regulatory progress sets the stage for 2017, as we are getting closer to establishing NBTXR3 in combination with radiotherapy as a new treatment modality for patients suffering from solid cancers.”
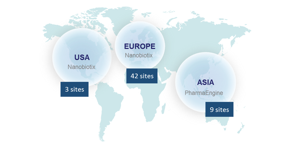
Global development of NBTXR3
The Company currently has seven ongoing clinical trials across the world. Overall 15 countries with 54 clinical centers and more than 300 physicians are involved in Nanobiotix’s clinical trials.
Clinical development of NBTXR3 by indication
NBTXR3 is being evaluated in: soft tissue sarcoma (STS), head and neck cancers, prostate cancer, and liver cancers (primary and metastases). Additionally, head and neck cancer and rectal cancer trials led by Nanobiotix’s Taiwanese partner, PharmaEngine, are underway in the Asia Pacific region.
Highlights of clinical news:
115 patients have been randomized and 153 have signed the informed consent in the STS pivotal clinical trial. The target of 104 patients needed for the interim readout has been reached. A total of 156 STS evaluable patients is expected in the “act.in.sarc” Phase II/III trial (www.actinsarc.com).
The Company expects to release the conclusion of the interim analysis conducted by an independent committee of experts in the coming months. This analysis will be performed four months after the 104th patient has been randomized (time to complete treatment plus readout).
PharmaEngine has started a new clinical trial in Asia in October 2016 for head and neck patient’s population receiving chemotherapy in combination with radiotherapy.
A Phase I/II prostate cancer trial has been initiated in the US and now recruiting at Ronald Reagan UCLA Medical Center, Los Angeles and Thomas Jefferson University Hospital, Philadelphia. Dana Farber Cancer Institute, Boston should be joining the trial soon.
Status of ongoing clinical trials
NBTXR3 first market authorization filing
The Company filed for certification of NBTXR3 in August 2016 based on the current level of clinical and scientific evidence. LNE/G-MED, the French notified body, has given guidance that the review of results for a potential CE mark is expected in 2017.
Expansion into immuno-oncology
In November, the Company presented preclinical data at the annual meeting of the Society for Immunotherapy of Cancer (SITC), demonstrating that NBTXR3 actively stimulates the host immune system to attack tumor cells. Study results suggested NBTXR3’s potential to transform the tumor into an in situ vaccine.
On top of the Company’s core development activities, these findings could open new potential collaborations for NBTXR3 through combinations with other immuno-oncology drugs.
Latest publications
Clinical Cancer Research – on NBTXR3 in Soft Tissue Sarcoma Phase I/II Trial: “First human study testing a new concept of radio enhancement using nanoparticles (NBTXR3) activated by radiation therapy in patients with locally advanced soft tissue sarcomas (STS)”, published 6 October 2016. Sylvie Bonvalot, Cécile Le Pechoux, Thierry Debaere, Guy Kantor, Xavier Buy, Eberhard Stoeckle, Paul Sargos, Philippe Terrier, Jean-michel Coindre, Nathalie Lassau, Rafik AIT SARKOUH, Mikaela Dimitriu, Elsa Borghi, Laurent Levy, Eric Deutsch and Jean Charles Soria. 10.1158/1078-0432.CCR-16-1297
Poster presented at SITC conference “Hafnium oxide nanoparticles, a radiation enhancer for in situ cancer vaccine”, authored by Paris S., Pottier A., Levy L., and Lu B.
####
About Nanobiotix
Nanobiotix (Euronext: NANO / ISIN: FR0011341205) is a late clinical-stage nanomedicine company pioneering novel approaches for the local treatment of cancer. The Company’s first-in-class, proprietary technology, NanoXray, enhances radiotherapy energy with a view to provide a new, more efficient treatment for cancer patients.
NanoXray products are compatible with current radiotherapy treatments and are meant to treat potentially a wide variety of solid tumors including soft tissue sarcoma, head and neck cancers, liver cancers, prostate cancer, breast cancer, glioblastoma, etc., via multiple routes of administration.
NBTXR3 is being evaluated in: soft tissue sarcoma (STS), head and neck cancers, prostate cancer, and liver cancers (primary and metastases). Additionally, head and neck cancer and rectal cancer trials led by Nanobiotix’s Taiwanese partner, PharmaEngine, are underway in the Asia Pacific region. The Company has filed in August 2016 for market approval (CE Marking) in Europe for its lead product NBTXR3.
Nanobiotix is listed on the regulated market of Euronext in Paris (ISIN: FR0011341205, Euronext ticker: NANO, Bloomberg: NANO: FP). The Company Headquarter is based in Paris, France. Affiliate in Cambridge, United States.
This press release contains certain forward-looking statements concerning Nanobiotix and its business. Such forward-looking statements are based on assumptions that Nanobiotix considers to be reasonable. However, there can be no assurance that the estimates contained in such forward-looking statements will be verified, which estimates are subject to numerous risks including the risks set forth in the reference document of Nanobiotix filed with the French Financial Markets Authority (Autorité des Marchés Financiers) under number D.16-0732 on July 22, 2016 (a copy of which is available on www.nanobiotix.com) and to the development of economic conditions, financial markets and the markets in which Nanobiotix operates. The forward-looking statements contained in this press release are also subject to risks not yet known to Nanobiotix or not currently considered material by Nanobiotix. The occurrence of all or part of such risks could cause actual results, financial conditions, performance or achievements of Nanobiotix to be materially different from such forward-looking statements.
This press release and the information that it contains do not constitute an offer to sell or subscribe for, or a solicitation of an offer to purchase or subscribe for, Nanobiotix shares in any country.
For more information, please click here
Contacts:
Nanobiotix
Sarah Gaubert
Head of Communication and Public Affairs
+33 (0)1 40 26 07 55
Media Relations - France
Springbok Consultants
Marina Rosoff
+33 (0)6 71 58 00 34
Media Relations - EU Outside France
Instinctif Partners
Melanie Toyne Sewell
+44 (0) 207 457 2020
Media Relations - United States
The Ruth Group
Kirsten Thomas / Chris Hippolyte
+1 508-280-6592 / +1 646-536-7023
Copyright © Nanobiotix
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








