Home > Press > Proton pinball on the catalyst: Moisture helps catalyst in fuel cells
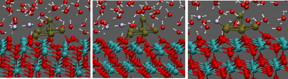 |
| Water on catalyst. CREDIT: CNR IOM/SISSA |
Abstract:
The function of fuel cells is to transform chemical energy into electricity through a chemical reaction. When this technology is mature enough it will be possible to use a fuel like hydrogen without emitting CO2 into the atmosphere. In the fuel cell, the chemical reaction is facilitated by a catalyst, typically platinum nanoparticles dispersed onto the surface of a durable and reactive material, such as cerium oxide, for example. Before this study, the active areas of these catalysts had been studied under ideal conditions, at very low temperatures and pressures, removing any dirt and moisture which could be found in the devices under ordinary working conditions. Stefano Fabris, a Physicist at the International School for Advanced Studies (SISSA) of Trieste and CNR-IOM Istituto Officina dei Materiali, and colleagues, however, wanted to study a system in realistic conditions, in this case adding a thin layer of water onto the catalyst. The team made some interesting discoveries: it seems the moisture, rather than making the processes less efficient, gives atoms in transit a "boost" thus significantly improving the overall efficiency of the system. The study, coordinated by Fabris, was published in the Journal of the American Chemical Society.
Proton pinball on the catalyst: Moisture helps catalyst in fuel cells
Trieste, Italy | Posted on August 3rd, 2016Fabris and colleagues' work is based on computer simulations. "This is a not an insignificant aspect, because traditional experimental techniques do not allow us to obtain detailed information about what happens at the interface between the surface of the catalyst and a liquid such as water. In this way, the atomic layers that separate the solid and the water remain a largely unexplored world, as difficult to measure as the core of a planet," explains Fabris. "The pressure and temperature conditions prevent a direct view at the experimental level. We must therefore find other ways to investigate this kind of phenomena, such as using these numerical simulations."
Chain Reaction
Fabris and colleagues reconstructed the physical system in detail, exactly where the surface of the catalyst comes into contact with one or more layers of water molecules and observed its evolution in real time. "First, we noticed that the water in contact with the catalyst breaks down, in part, into hydrogen ions, or protons, and hydroxide ions (OH-).
This was not completely unexpected, says Matteo Farnesi Camellone, CNR-IOM (Istituto Officina dei Materiali) Researcher and first author of the work, adding that an effect like this could have been imagined a priori. "The really interesting part happens after this breakdown," says Farnesi Camellone. When there is a certain number of protons and hydroxide ions on the surface, a so-called proton chain occurs: "a sort of pinball game where the OH- groups pass a free proton back and forth incessantly, binding it and releasing it. In the process water molecules form and break up continuously, while the protons continue to bounce and travel long distances along the surface." The consequences for the catalytic process are positive. "All of this movement helps transport molecules between the active zones of the material. We measured increases in transport and release speed several times, the efficiency of the catalyst actually improves."
"This is the first time the catalyst has been studied with water present. Our study, besides showing that the process is favored by moisture, goes beyond to explain what happens in the material in detail, which is important knowledge for designing better fuel cells, "says Fabris.
####
For more information, please click here
Contacts:
Federica Sgorbissa
39-040-378-7644
Copyright © International School of Advanced Studies (SISSA)
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








