Home > Press > A protective shield for sensitive catalysts: Hydrogels block harmful oxygen
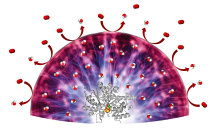 |
| With a novel hydrogel, sensitive catalysts can be protected from oxygen molecules (red) which could irreversibly damage the catalysts. The hydrogel converts oxygen into water (red-white).
© Felipe Conzuelo |
Abstract:
An international research team has found a way of protecting sensitive catalysts from oxygen-caused damage. In the future, this could facilitate the creation of hydrogen fuel cells with molecular catalysts or with biomolecules such as the hydrogenase enzyme. To date, this could only be accomplished using the rare and expensive precious metal platinum. Together with their French colleagues, researchers from Bochum and Mülheim describe the way in which a hydrogel can serve as a "protective shield" for biomolecules by two articles written in the journals Angewandte Chemie and the Journal of the American Chemical Society.
A protective shield for sensitive catalysts: Hydrogels block harmful oxygen
Bochum, Germany | Posted on June 15th, 2015Requirements on catalysts are difficult to reconcile
In order to be suitable for industrial applications, catalysts have to be efficient, stable and affordable; in addition, they have to be tailor-cut for one specific chemical reaction. "Uniting all of these requirements in one molecule is a considerable challenge," says Dr Nicolas Plumeré from the Chemistry Department at the Ruhr-Universität Bochum. However, a novel hydrogel in which catalysts are embedded could greatly simplify the development of fuel cell catalysts in the future. To explore this possibility, the researchers from Bochum began a collaborative project with colleagues from the Max Planck Institute for Chemical Energy Conversion in Mülheim and from Aix Marseille University and the Centre National de la Recherche Scientifique (CNRS) in France.
Hydrogel acting as solvent and as protective environment
For their experiments, the German team utilised the hydrogenase enzyme from the green alga Chlamydomonas rheinhardtii; it splits hydrogen into protons and electrons. Typically, even trace amounts of oxygen cause irreversible damage to this biomolecule. However, the researchers incorporated it in a hydrogel which assumes two functions: it acts as a solvent, ensuring that all reaction partners reach the enzyme quickly and easily. At the same time, it provides a protective environment in which the oxygen cannot penetrate through to the enzyme, even if it is present at relatively high concentrations. The trick: the hydrogenase activity leads to the creation of electrons; they wander through the hydrogel and are transmitted to the oxygen, thus converting it into a harmless form, namely water.
Catalyst design could become considerably easier in the future
Using simulations and experiments, the German-French team demonstrated another important property of hydrogels. The activity of many catalysts decreases over time due to exposure to deactivating molecules. Some can be rendered functional again through special reactivation processes. Notably, however, the hydrogel protects even those catalysts for which a reactivation process does not exist. "In future, we will thus no longer have to pay attention to the robustness or suitable reactivation processes when developing catalysts for technical applications," explains Olaf Rüdiger, Chemist at the Max Planck Institute for Chemical Energy Conversion. "We can focus solely on maximising the catalyst's activity. This will simplify the development process to a considerable degree and open up new possibilities for the manufacture of fuel cells."
###
Funding
The German Research Foundation funded the project as part of the RESOLV Cluster of Excellence (EXC 1069). The French subproject has been carried out thanks to the support of the A*MIDEX project "MicrobioE" (n° ANR-11-IDEX-0001-02) funded by the "Investissements d'Avenir" French Government programme.
Bibliographic record
A. Alsheikh Oughli, F. Conzuelo, M. Winkler, T. Happe, W. Lubitz, W. Schuhmann, O. Rüdiger, N. Plumeré (2015): Protection from oxidative damage of the O2 sensitive [FeFe]-hydrogenase from Chlamydomonas reinhardtii using a redox hydrogel, Angewandte Chemie International Edition, DOI: 10.1002/anie.201502776R1
V. Fourmond, S. Stapf, H. Li, D. Buesen, J. Birrell, R. Olaf; W. Lubitz, W. Schuhmann, N. Plumeré, C. Léger (2015): The mechanism of protection of catalysts supported in redox hydrogel films, Journal of the American Chemical Society, DOI: 10.1021/jacs.5b01194
####
For more information, please click here
Contacts:
Dr Nicolas Plumeré
junior research team
Molecular Nanostructures at the Centre for Electrochemical Sciences (CES)
Faculty of Chemistry and Biochemistry
Ruhr-Universität
44780 Bochum, Germany
phone: +49/234/32-29434
Dr Olaf Rüdiger
head of research group
Protein-Electrochemistry
Max Planck Institute for Chemical Energy Conversion
Mülheim an der Ruhr, Germany
phone: +49/208/306 3526
Copyright © Ruhr University Bochum
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Hydrogels
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
![]() Shrinking hydrogels enlarge nanofabrication options: Researchers from Pittsburgh and Hong Kong print intricate, 2D and 3D patterns December 29th, 2022
Shrinking hydrogels enlarge nanofabrication options: Researchers from Pittsburgh and Hong Kong print intricate, 2D and 3D patterns December 29th, 2022
![]() The deformation of the hydrogel is used to measure the negative pressure of water April 22nd, 2022
The deformation of the hydrogel is used to measure the negative pressure of water April 22nd, 2022
![]() Nanocellulose decorated with proteins is suitable for 3D cell culturing September 24th, 2021
Nanocellulose decorated with proteins is suitable for 3D cell culturing September 24th, 2021
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








