Home > Press > Up in Flames: Evidence Confirms Combustion Theory: Berkeley Lab and University of Hawaii research outlines the story of soot, with implications for cleaner-burning fuels
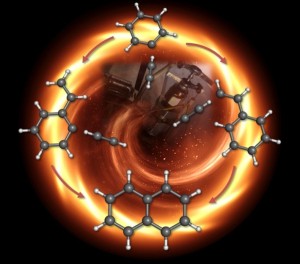 |
| Graphical representation of the chemistry in the early stages of soot formation. The mechanism to the right was demonstrated by experiment, while the one on the left was not. Credit: Dorian Parker, University of Hawaii |
Abstract:
Researchers at the Department of Energy's Lawrence Berkeley National Lab (Berkeley Lab) and the University of Hawaii have uncovered the first step in the process that transforms gas-phase molecules into solid particles like soot and other carbon-based compounds.
Up in Flames: Evidence Confirms Combustion Theory: Berkeley Lab and University of Hawaii research outlines the story of soot, with implications for cleaner-burning fuels
Berkeley, CA | Posted on July 1st, 2014The finding could help combustion chemists make more-efficient, less-polluting fuels and help materials scientists fine-tune their carbon nanotubes and graphene sheets for faster, smaller electronics. In addition, the results could have implications for the burgeoning field of astrochemistry, potentially establishing the chemical process for how gaseous outflows from stars turn into carbon-based matter in space.
"When you burn a flame, you start with a gas-phase reactant and then analyze the products, which include soot," says Musahid Ahmed, scientist in the Chemical Sciences Division at Berkeley Lab. "But there is no direct evidence for the chemical bonds that break and form in the process." For more than 30 years, scientists have developed computational models of combustion to explain how gas molecules form soot, but now Ahmed and his colleagues have data to confirm one long-standing theory in particular. "Our paper presents the first direct observation of this process," he says.
While the research is relevant to a number of disciplines—combustion science, materials science, and astrochemistry—it's combustion science that could see the most direct impact the soonest, says Ahmed. Specifically, the fundamental chemistry discovery could be used to find or design fuels that burn cleaner and don't produce as much soot.
Think about your car engine. If the combustion process were perfect, only carbon dioxide and water would come out of the tailpipe. Instead, we see fumes and particulates like soot, a visible macromolecule made up of sheets of carbon.
Theoretically, there are hundreds of different ways molecules can combine to create these dirty emissions. But there has been one popular class of mechanisms that outlines possible early steps for bond making and bond breaking during combustion. Called hydrogen abstraction-acetylene addition, or HACA, it was developed by Michael Frenklach professor of mechanical engineering at the University of California Berkeley in 1991.
One version of HACA works like this: during the high-temperature, high-pressure environment of combustion, a simple ring of six carbon and six hydrogen atoms, called benzene, would lose one of its hydrogen atoms, allowing another two-carbon molecule called acetylene, to attach to the ring, giving it a kind of tail. Then the acetylene tail would lose one of its hydrogen atoms so another acetylene could link up in, doubling the carbon atoms in the tail to four.
Next, the tail would curl around and attach to the original ring, creating a double-ring structure called naphthalene. Link by link, ring by ring, these molecules would continue to grow in an unwieldy, crumpled way until they became the macromolecules that we recognize as soot.
To test the first step of the theoretical HACA mechanism, Ahmed and collaborators from the University of Hawaii used a beamline at the Advanced Light Source (ALS) at Berkeley Lab specifically outfitted to study chemical dynamics. The ALS, a DOE Office of Science user facility, produces numerous photons over a wide range of energies, allowing researchers to probe a variety of molecules produced in this chemical reaction with specialized mass spectrometry analysis.
Unique to this experimental setup, Ahmed's team used a so-called hot nozzle, which recreates combustion environment in terms of pressure and temperature. The group started with a gaseous mix of nitrosobenzene (a benzene ring with a molecule of nitrogen and oxygen attached) and acetylene, and pumped it through a heated tube at a pressure of about 300 torr and a temperature of about 750 degrees Celsius. The molecules that came out the other end were immediately skimmed into a mass spectrometer that made use of the synchrotron light for analysis.
The researchers found two molecules predominantly emerged from the process. The more abundant kind was the carbon ring with a short acetylene tail on it, called phenylacetylene. But they also saw evidence for the double ring, naphthalene. These results, says Ahmed, effectively rule out one HACA mechanism—that a carbon ring would gain two separate tails and those tails would bond to form the double ring—and confirm the most popular HACA mechanism where a long tail curls around to form naphthalene.
Ahmed's local team included Tyler Troy, postdoctoral fellow at Berkeley Lab, and this work was performed with long-term collaborator Ralf Kaiser, professor of physical chemistry at the University of Hawaii at Manoa, and Dorian Parker, postdoctoral fellow also at Hawaii. The research was published June 20 online in the journal Angewandte Chemie.
"Having established the route to naphthalene, the simplest polycyclic aromatic hydrocarbon, the next step will be to unravel the pathways to more complex systems," says Kaiser.
Further experiments will investigate these follow-up mechanisms. It's a tricky feat, explains Ahmed, because the molecular possibilities quickly multiply. The researchers will add infrared spectroscopy to their analysis in order to catch the variety of molecules that form during these next phases of combustion.
This research was funded by the DOE Office of Science.
####
About DOE/Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
The DOE Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.
For more information, please click here
Contacts:
Kate Greene
510-486-4404
Copyright © DOE/Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() The chemical dynamics beamline can be viewed on the web at:
The chemical dynamics beamline can be viewed on the web at:
![]() Snapshots of Ahmed’s science are available at:
Snapshots of Ahmed’s science are available at:
![]() Prof. Kaiser’s homepage is here:
Prof. Kaiser’s homepage is here:
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Graphene/ Graphite
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings/Nanosheets
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Environment
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Automotive/Transportation
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Aerospace/Space
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Research partnerships
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








