Home > Press > An Organized Approach to 3D Tissue Engineering: IBN’s novel technique brings researchers closer to viable organ implants
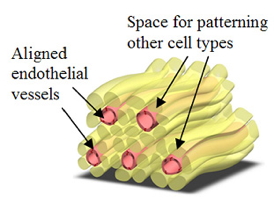 |
| Schematic diagram illustrating the concept of a prevascularized hydrogel. The adjacent fibers could be used to pattern other cell types around the vessels. |
Abstract:
Researchers at the Institute of Bioengineering and Nanotechnology (IBN) have developed a simple method of organizing cells and their microenvironments in hydrogel fibers. Their unique technology provides a feasible template for assembling complex structures, such as liver and fat tissues, as described in their recent publication in Nature Communications1.
An Organized Approach to 3D Tissue Engineering: IBN’s novel technique brings researchers closer to viable organ implants
Singapore | Posted on August 21st, 2013According to IBN Executive Director Professor Jackie Y. Ying, "Our tissue engineering approach gives researchers great control and flexibility over the arrangement of individual cell types, making it possible to engineer prevascularized tissue constructs easily. This innovation brings us a step closer toward developing viable tissue or organ replacements."
IBN Team Leader and Principal Research Scientist, Dr Andrew Wan, elaborated, "Critical to the success of an implant is its ability to rapidly integrate with the patient's circulatory system. This is essential for the survival of cells within the implant, as it would ensure timely access to oxygen and essential nutrients, as well as the removal of metabolic waste products. Integration would also facilitate signaling between the cells and blood vessels, which is important for tissue development."
Tissues designed with pre-formed vascular networks are known to promote rapid vascular integration with the host. Generally, prevascularization has been achieved by seeding or encapsulating endothelial cells, which line the interior surfaces of blood vessels, with other cell types. In many of these approaches, the eventual distribution of vessels within a thick structure is reliant on in vitro cellular infiltration and self-organization of the cell mixture. These are slow processes, often leading to a non-uniform network of vessels within the tissue. As vascular self-assembly requires a large concentration of endothelial cells, this method also severely restricts the number of other cells that may be co-cultured.
Alternatively, scientists have attempted to direct the distribution of newly formed vessels via three-dimensional (3D) co-patterning of endothelial cells with other cell types in a hydrogel. This approach allows large concentrations of endothelial cells to be positioned in specific regions within the tissue, leaving the rest of the construct available for other cell types. The hydrogel also acts as a reservoir of nutrients for the encapsulated cells. However, co-patterning multiple cell types within a hydrogel is not easy. Conventional techniques, such as micromolding and organ printing, are limited by slow cell assembly, large volumes of cell suspension, complicated multi-step processes and expensive instruments. These factors also make it difficult to scale up the production of implantable 3D cell-patterned constructs. To date, these approaches have been unsuccessful in achieving vascularization and mass transport through thick engineered tissues.
To overcome these limitations, IBN researchers have used interfacial polyelectrolyte complexation (IPC) fiber assembly, a unique cell patterning technology patented by IBN, to produce cell-laden hydrogel fibers under aqueous conditions at room temperature. Unlike other methods, IBN's novel technique allows researchers to incorporate different cell types separately into different fibers, and these cell-laden fibers may then be assembled into more complex constructs with hierarchical tissue structures. In addition, IBN researchers are able to tailor the microenvironment for each cell type for optimal functionality by incorporating the appropriate factors, e.g. proteins, into the fibers. Using IPC fiber assembly, the researchers have engineered an endothelial vessel network, as well as cell-patterned fat and liver tissue constructs, which have successfully integrated with the host circulatory system in a mouse model and produced vascularized tissues.
The IBN researchers are now working on applying and further developing their technology toward engineering functional tissues and clinical applications.
Reference:
1. M. F. Leong, J. K. C. Toh, D. Chan, K. Narayanan, H. F. Lu, T. C. Lim, A. C. A. Wan and J. Y. Ying, "Patterned Prevascularized Tissue Constructs by Assembly of Polyelectrolyte Hydrogel Fibers," Nature Communications, (2013) DOI: 10.1038/ncomms3353.
####
About Institute of Bioengineering and Nanotechnology
The Institute of Bioengineering and Nanotechnology (IBN) was established in 2003 and is spearheaded by its Executive Director, Professor Jackie Yi-Ru Ying.
Professor Ying was a Professor of Chemical Engineering at the Massachusetts Institute of Technology (1992 - 2005). She was recognized as one of “One Hundred Engineers of the Modern Era” by the American Institute of Chemical Engineers in 2008 for her groundbreaking work on nanostructured systems, nanoporous materials and host matrices for quantum dots and wires.
Under her direction, IBN conducts research at the cutting-edge of bioengineering and nanotechnology. Its programs are geared towards linking multiple disciplines across engineering, science and medicine to produce research breakthroughs that will improve healthcare and our quality of life.
IBN’s research activities are focused in the following areas:
Nanomedicine, where functionalized polymers, hydrogels and biologics are developed as therapeutics and carriers for the controlled release and targeted delivery of therapeutics to diseased cells and organs.
Cell and Tissue Engineering, where biomimicking materials, stem cell technology, microfluidic systems and bioimaging tools are combined to develop novel approaches to regenerative medicine and artificial organs.
Biodevices and Diagnostics, which involve nanotechnology and microfabricated platforms for high-throughput biomarker and drug screening, automated biologics synthesis, and rapid disease diagnosis.
Green Chemistry and Energy, which encompass the green synthesis of chemicals and pharmaceuticals, catalytic conversion of biomass, utilization of carbon dioxide, and new nanocomposite materials for energy applications.
IBN's innovative research is aimed at creating new knowledge and intellectual properties in the emerging fields of bioengineering and nanotechnology to attract top-notch researchers and business partners to Singapore. Since 2003, IBN researchers have published over 880 papers in leading journals.
IBN also plays an active role in technology transfer and spinning off companies, linking the research institute and industrial partners to other global institutions. The Institute has a portfolio of over 620 patents/patent applications, and welcomes industrial and clinical partners to collaborate on and co-develop its technologies. IBN has successfully commercialized 50 patents/patent applications, and has established 7 spin-off companies.
IBN's current staff and students strength stands at over 155 scientists, engineers and medical doctors. With its multinational and multidisciplinary research staff, the institute is geared towards generating new biomaterials, devices, systems and processes to boost Singapore’s economy in the medical technology, pharmaceuticals, chemicals, consumer products and clean technology sectors.
IBN is also committed to nurturing young talents. Besides the training of PhD students, IBN has a Youth Research Program (YRP) for students and teachers from secondary schools, junior colleges, polytechnics, and universities. Since its inception in October 2003, YRP has reached out to more than 64,500 students and teachers from 290 local and overseas schools and institutions. Over 1,770 students and teachers have completed research attachments at IBN for a minimum period of four weeks.
For more information, visit www.ibn.a-star.edu.sg.
For more information, please click here
Contacts:
Elena Tan
Phone: 65 6824 7032
Nidyah Sani
65-682-47005
Copyright © Institute of Bioengineering and Nanotechnology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








