Home > Press > Giving transplanted cells a nanotech checkup: Researchers devise a way to safely see whether replacement cells are still alive
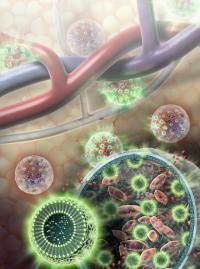 |
| Nanosensors (green spheres) are composed of fat and L-arginine molecules, as well as separate indicator molecules that give off MRI-detectable and light signals when cells are alive. Nanosensors are enclosed in a hydrogel membrane along with liver cells (pink). Nutrients and other relatively small molecules (red) are able to travel across the hydrogel membrane to and from the bloodstream.
Credit: Sayo Studios |
Abstract:
Researchers at Johns Hopkins have devised a way to detect whether cells previously transplanted into a living animal are alive or dead, an innovation they say is likely to speed the development of cell replacement therapies for conditions such as liver failure and type 1 diabetes. As reported in the March issue of Nature Materials, the study used nanoscale pH sensors and magnetic resonance imaging (MRI) machines to tell if liver cells injected into mice survived over time.
Giving transplanted cells a nanotech checkup: Researchers devise a way to safely see whether replacement cells are still alive
Baltimore, MD | Posted on February 5th, 2013"This technology has the potential to turn the human body into less of a black box and tell us if transplanted cells are still alive," says Mike McMahon, Ph.D., an associate professor of radiology at the Johns Hopkins University School of Medicine who oversaw the study. "That information will be invaluable in fine-tuning therapies."
Regenerative medicine advances depend on reliable means of replacing damaged or missing cells, such as injecting pancreatic cells in people with diabetes whose own cells don't make enough insulin. To protect the transplanted cells from the immune system, while allowing the free flow of nutrients and insulin between the cells and the body, they can be encased in squishy hydrogel membranes before transplantation. But, explains McMahon, "once you put the cells in, you really have no idea how long they survive." Such transplanted cells eventually stop working in most patients, who must resume taking insulin. At that point, physicians can only assume that cells have died, but they don't know when or why, says McMahon.
With that problem in mind, McMahon's group, which specializes in methods of detecting chemical changes, collaborated with the research group headed by Jeff Bulte, Ph.D., the director of cellular imaging at Hopkins' Institute for Cell Engineering. Bulte's group devises ways of tracking implanted cells through the body using MRI. Led by research fellow Kannie Chan, Ph.D., the team devised an extremely tiny, or nanoscale, sensor filled with L-arginine, a nutritional supplement that responds chemically to small changes in acidity (pH) caused by the death of nearby cells. Changes in the acidity would in turn set off changes in sensor molecules embedded in the thin layer of fat that makes up the outside of the nanoparticle, giving off a signal that could be detected by MRI.
To test how these nanosensors would work in a living body, the team loaded them into hydrogel spheres along with liver cells — a potential therapy for patients with liver failure — and another sensor that gives off bioluminescent light only while the cells are alive. The spheres were injected just under the skin of mice. As confirmed by the light signal, the MRI accurately detected where the cells were in the body and what proportion were still alive. (Such light indicators cannot be used to track cells in humans because our bodies are too large for visible signals to get through, but these indicators allowed the team to check whether the MRI nanosensors were working properly in the mice.)
"It was exciting to see that this works so well in a living body," Chan says. The team hopes that because the components of the system — hydrogel membrane, fat molecules, and L-arginine — are safe for humans, adapting their discovery for clinical use will prove relatively straightforward. "This should take a lot of the guesswork out of cell transplantation by letting doctors see whether the cells survive, and if not, when they die," Chan says. "That way they may be able to figure out what's killing the cells, and how to prevent it."
Potential applications of the sensors are not limited to cells inside hydrogel capsules, Bulte notes. "These nanoparticles would work outside capsules, and they could be paired with many different kinds of cells. For example, they may be used to see whether tumor cells are dying in response to chemotherapy," he says.
Other authors on the paper were Guanshu Liu, Xiaolei Song, Heechul Kim, Tao Yu, Dian R. Arifin, Assaf A. Gilad, Justin Hanes, Piotr Walczak and Peter C. M. van Zijl, all of the Johns Hopkins University School of Medicine.
The study was funded by the National Institute of Biomedical Imaging and Bioengineering (grant numbers R01 EB012590, EB015031, EB015032 and EB007825).
####
For more information, please click here
Contacts:
Shawna Williams
410-955-8236
Copyright © Johns Hopkins Medicine
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() New Technique Developed for Tracking Cells in the Body:
New Technique Developed for Tracking Cells in the Body:
![]() Tracking the Elusive Stem Cell:
Tracking the Elusive Stem Cell:
![]() Jeff Bulte on Tracking Cells Through the Body:
Jeff Bulte on Tracking Cells Through the Body:
![]() Hopkins Imaging Scientist Earns New NIH 'Eureka' Grant for Exceptional, Unconventional Research:
Hopkins Imaging Scientist Earns New NIH 'Eureka' Grant for Exceptional, Unconventional Research:
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Sensors
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








