Home > Press > Bejeweled: Nanotech gets boost from nanowire decorations
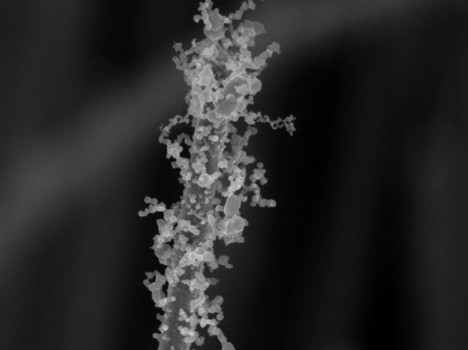 |
| Decoration with nanoparticles creates intricate surface patterns full of nooks and crannies, twists and turns that greatly improve surface area. Image courtesy of the Stanford Nanocharacterization Laboratory. |
Abstract:
Engineers at Stanford have found a novel method for "decorating" nanowires with chains of tiny particles to increase their electrical and catalytic performance. The new technique is simpler, faster and provides greater control than earlier methods and could lead to better batteries, solar cells and catalysts.
Bejeweled: Nanotech gets boost from nanowire decorations
Stanford, CA | Posted on April 27th, 2012Like a lead actress on the red carpet, nanowires—those superstars of nanotechnology—can be enhanced by a little jewelry, too. Not the diamonds and pearls variety, but the sort formed of sinuous chains of metal oxide or noble metal nanoparticles.
Though science has known for some time that such ornamentation can greatly increase the surface area and alter the surface chemistry of nanowires, engineers at Stanford University have found a novel and more effective method of "decorating" nanowires that is simpler and faster than previous techniques. The results of their study were published recently in the journal Nano Letters.
The development, say the researchers, might someday lead to better lithium-ion batteries, more efficient thin-film solar cells and improved catalysts that yield new synthetic fuels.
Tree-like structures
"You can think of it like a tree. The nanowires are the trunk, very good at transporting electrons, like sap, but limited in surface area," explained Xiaolin Zheng, an assistant professor of mechanical engineering and senior author of the study. "The added nanoparticle decorations, as we call them, are like the branches and leaves, which fan out and greatly increase the surface area."
At the nanoscale, surface area matters a great deal in engineering applications like solar cells, batteries and, especially catalysts, where the catalytic activity is dependent on the availability of active sites at the surface of the material.
"Greater surface area means greater opportunity for reactions and therefore better catalytic capabilities in, for example, water-splitting systems that produce clean-burning hydrogen fuel from sunlight," said Yunzhe Feng, a research assistant in Zheng's lab and first author of the study.
Other applications such as sensing small concentrations of chemicals in the air—of toxins or explosives, for example—might also benefit from the greater likelihood of detection made possible by increased surface area.
A spark of an idea
The key to the Stanford team's discovery was a flame. Engineers had long known that nanoparticles could be adhered to nanowires to increase surface area, but the methods for creating them were not very effective in forming the much-desired porous nanoparticle chain structures. These other methods proved too slow and resulted in a too-dense, thick layer of nanoparticles coating the wires, doing little to increase the surface area.
Zheng and her team wondered whether a quick burst of flame might work better, so they tried it.
Zheng dipped the nanowires in a solvent-based gel of metal and salt, then air-dried them before applying the flame. In her process the solvent burns away in a few seconds, allowing the all-important nanoparticles to crystalize into branch-like structures fanning out from the nanowires.
"We were a little surprised by how well it worked," said Zheng. "It performed beautifully."
Using sophisticated microscopes and spectroscopes at the Stanford Nanocharacterization Laboratory, the engineers were able to get a good look at their creations.
"It created these intricate, hair-like tendrils filled with lots of nooks and crannies," said Zheng. The bejeweled nanowires look like pipe cleaners. The resulting structure increases the surface many fold over what went before, she said.
Dramatic performance, unprecedented control
"The performance improvements have so far been dramatic," said In Sun Cho, a post-doctoral fellow in Zheng's lab and co-author of the paper.
Zheng and team have dubbed the technique the sol-flame method, for the combination of solvent and flame that yields the nanoparticle structures. The method appears general enough to work with many nanowire and nanoparticle materials and, perhaps more importantly, provides an unprecedented degree of engineering control in creating the nanoparticle decorations.
The high temperature of the flame and brief annealing time ensure that the nanoparticles are small and spread evenly across the nanowires. And, by varying the concentration of nanoparticle in the precursor solution and the number of times the wires are dip-coated, the Stanford team was able to vary the size of the nanoparticle decorations from tens to hundreds of nanometers, and the density from tens to hundreds of particles per square micrometer.
"Though more research is needed, such precision is crucial and could bolster the wider adoption of the process," said Zheng.
Pratap M. Rao and Lili Cai also contributed to this research. The study was supported by the ONR/PECASE program.
Author Andrew Myers is associate director of communications for the Stanford School of Engineering.
####
For more information, please click here
Contacts:
Andrew Myers
650-736-2245
Copyright © Stanford University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
Thin films
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Solar/Photovoltaic
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
![]() KAIST researchers introduce new and improved, next-generation perovskite solar cell November 8th, 2024
KAIST researchers introduce new and improved, next-generation perovskite solar cell November 8th, 2024
![]() Groundbreaking precision in single-molecule optoelectronics August 16th, 2024
Groundbreaking precision in single-molecule optoelectronics August 16th, 2024
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








