Home > Press > On the Way to Hydrogen Storage? A magnesium hydride cluster as a model for a hydrogen storage material at the sub-nanometer level
 |
Abstract:
The car of the future could be propelled by a fuel cell powered with hydrogen. But what will the fuel tank look like? Hydrogen gas is not only explosive but also very space-consuming. Storage in the form of very dense solid metal hydrides is a particularly safe alternative that accommodates the gas in a manageable volume. As the storage tank should also not be too heavy and expensive, solid-state chemists worldwide focus on hydrides containing light and abundant metals like magnesium. Sjoerd Harder and his co-workers at the Universities of Groningen (Netherlands) and Duisburg-Essen (Germany) now take the molecular approach. As the researchers report in the journal Angewandte Chemie, extremely small clusters of molecular magnesium hydride could be a useful model substance for more precise studies about the processes involved in hydrogen storage.
On the Way to Hydrogen Storage? A magnesium hydride cluster as a model for a hydrogen storage material at the sub-nanometer level
Germany | Posted on April 19th, 2011Magnesium hydride (MgH2) can release hydrogen when needed and the resulting magnesium metal reacts back again to form the hydride by pressurizing with hydrogen at a "gas station". Unfortunately, this is an idealized picture. Not only is the speed of hydrogen release/uptake excessively slow (kinetics) but it also only operates at higher temperatures (thermodynamics). The hydrides, the negatively charged hydrogen atoms (H─), are bound so strongly in the crystal lattice of magnesium cations (Mg2+) that temperatures of more than 300 ˚C are needed to release the hydrogen gas.
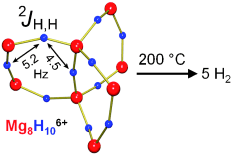
Particularly intensive milling has made it possible to obtain nanocrystalline materials, which, on account of its larger surface, rapidly release or take up hydrogen. However, the high stability of the magnesium hydride still translates to rather high release temperatures. According to recent computer calculations, magnesium hydride clusters of only a few atoms possibly could generate hydrogen at temperatures far below 300 °C. Clusters with less than 20 Mg2+ ions are smaller than one nanometer and behave differently from the bulk material. Their hydride ions have fewer Mg2+ neighbors and are more weakly bound. However, it is extremely difficult to obtain such tiny clusters by milling. In Harder's "bottom-up" approach, magnesium hydride clusters are made by starting from molecules. The challenge is to prevent such clusters from forming very stable bulk material. Using a special ligand system, they could trap a cluster that resembles a paddle wheel made of eight Mg2+ and ten H─ ions. For the first time it was shown that molecular clusters indeed release hydrogen already at the temperature of 200 °C.
This largest magnesium hydride cluster reported to date is not practical for efficient hydrogen storage but shines new light on a current problem. It is easily studied by molecular methods and as a model system could provide detailed insights in hydrogen storage.
####
For more information, please click here
Contacts:
Sjoerd Harder
University of Groningen (Netherlands)
H. H. (Hilda) Biemold +31 50 363 4235
Postal address Stratingh Institute for Chemistry
University of Groningen
Nijenborgh 4
NL-9747 AG Groningen
The Netherlands
E-mail
+31-50-363 4322
FAX +31 50 363 4296
Copyright © Wiley-VCH Verlag GmbH & Co. KGaA
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








