Home > Press > Researchers show how cells open 'doors' to release neurotransmitters
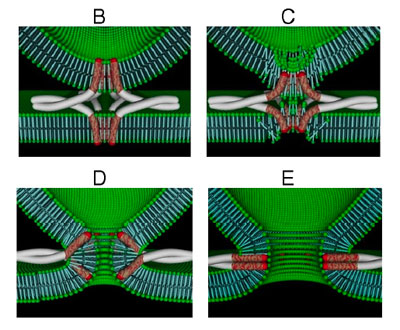 |
| A schematic model of a fusion pore opening. |
Abstract:
Like opening a door to exit a room, cells in the body open up their outer membranes to release such chemicals as neurotransmitters and other hormones.
Cornell researchers have shed new light on this lightning-quick, impossibly small-scale process, called exocytosis, by casting sharp focus on what happens right at the moment the "doors" on the cell wall open.
By Anne Ju
Researchers show how cells open 'doors' to release neurotransmitters
Ithaca, NY | Posted on October 13th, 2010Publishing online Oct. 11 in Proceedings of the National Academy of Sciences, researchers led by Cornell's Manfred Lindau used a combination of molecular biology, electrophysiology, microfabricated electrochemical sensors and advanced microscopy to elucidate exocytosis of noradrenaline. This is a neurotransmitter released from the adrenal gland by a type of neuroendocrine cell called a chromaffin cell.
Lindau, professor of applied and engineering physics, studies the properties of exocytosis by looking at how packets of chemicals called vesicles adhere to the cell wall and open the door between the vesicle interior and cell's exterior. This "door" is called the fusion pore.
"Biochemists have been working on experiments to identify what proteins and molecules are the main players in this mechanism of release," Lindau said.
It turns out that neurotransmitter release is largely regulated by a set of proteins called SNARE proteins, and one called synaptobrevin is located on the cell's vesicle membrane. The synaptobrevins bind with other proteins called syntaxin and SNAP-25, which are located in the plasma membrane that encloses the cell. When the cell is excited and the neurotransmitter release is triggered, these proteins together are believed to open the fusion pore.
Lindau's team of researchers used genetically altered mouse embryos that lacked synaptobrevin and introduced viruses with modified versions of the protein into their experiments. They then imaged and studied the release function of the cells for the different versions of the synaptobrevins.
They discovered that one end of the synaptobrevin -- the part that anchors it in the vesicle membrane -- is pulled deeper into the vesicle membrane when the cell is stimulated. This movement is what temporarily changes the structure of the membrane and allows the opening of the fusion pore and neurotransmitter release. It had previously been thought that the fusion pore originates by an indirect effect of the SNARE protein in the membrane lipids. Lindau's experiments have shown that the vesicle membrane component of synaptobrevin is the active part of the molecular nanomachine that forms the fusion pore.
To continue visualizing the molecular details of this complex process, Lindau is working on sabbatical at the University of Oxford with professor Mark Sansom on molecular dynamics and computer simulations of the SNARE proteins and neurotransmitter exocytosis.
The research published in PNAS was funded primarily by the National Institutes of Health and the Cornell Nanobiotechnology Center, which is supported by the National Science Foundation. The paper's authors include former Cornell graduate students Annita Ngatchou and Kassandra Kisler, postdoctoral associates Qinghua Fang and Yong Zhao, and collaborators from the Max Planck Institute for Biophysical Chemistry, the University of Saarland in Germany and the University of Copenhagen.
####
For more information, please click here
Contacts:
Media Contact:
Joe Schwartz
(607) 254-6235
Cornell Chronicle:
Anne Ju
(607) 255-9735
Copyright © Cornell University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Academic/Education
![]() Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
Rice University launches Rice Synthetic Biology Institute to improve lives January 12th, 2024
![]() Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Multi-institution, $4.6 million NSF grant to fund nanotechnology training September 9th, 2022
Molecular Machines
![]() First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
First electric nanomotor made from DNA material: Synthetic rotary motors at the nanoscale perform mechanical work July 22nd, 2022
![]() Nanotech scientists create world's smallest origami bird March 17th, 2021
Nanotech scientists create world's smallest origami bird March 17th, 2021
![]() Giant nanomachine aids the immune system: Theoretical chemistry August 28th, 2020
Giant nanomachine aids the immune system: Theoretical chemistry August 28th, 2020
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








