Home > Press > For platinum catalysts, smaller may be better
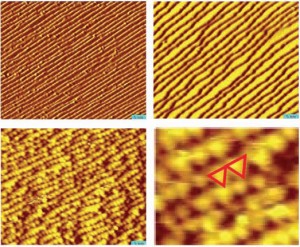 |
| In these STM images of a platinum catalyst, (A) shows the terraced the surface under ultrahigh vacuum, (B) as the surface is covered with carbon monoxide and pressure increases, the terraces widen (C) when coverage is complete and press reaches one torr, the terraces fracture into nanoclusters (D) enlarged view shows triangular shape of the nanoclusters, two of which are marked by red lines. |
Abstract:
When it comes to metal catalysts, the platinum standard is, well, platinum! However, at about $2,000 an ounce, platinum is more expensive than gold. The high cost of the raw material presents major challenges for the future wide scale use of platinum in fuel cells. Research at the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) suggests that one possible way to meet these challenges is to think small - really small.
For platinum catalysts, smaller may be better
Berkeley, CA | Posted on June 30th, 2010A study led by Gabor Somorjai and Miquel Salmeron of Berkeley Lab's Materials Sciences Division showed that under high pressure, comparable to the pressures at which many industrial technologies operate, nanoparticle clusters of platinum potentially can out-perform the single crystals of platinum now used in fuel cells and catalytic converters.
"We've discovered that the presence of carbon monoxide molecules can reversibly alter the catalytic surfaces of platinum single crystals, supposedly the most thermodynamically stable configuration for a platinum catalyst," said Somorjai, one of the world's foremost experts on surface chemistry and catalysis. "This indicates that under high-pressure conditions, single crystals of platinum are not as stable as nanoclusters, which actually become more stabilized as carbon monoxide molecules are co-adsorbed together with platinum atoms."
"Our results also demonstrate that the limitations of traditional surface science techniques can be overcome with the use of techniques that operate under realistic conditions, says Salmeron, a leading authority on surface imaging and developer of the in situ imaging and spectroscopic techniques used in this study. He is also the director of Berkeley Lab's Materials Sciences Division.
In this study, single crystal platinum surfaces were examined under high-pressure. The surfaces were structured as a series of flat terraces about six atoms wide separated by atomic steps. Such structural feature are common in metal catalysts and are considered to be the active sites where catalytic reactions occur. Single crystals are used as models for these features.
Somorjai and Salmeron coated the platinum surfaces in this study with carbon monoxide gas, a reactant involved in many important industrial catalytic processes, including the Fischer-Tropsch process for making liquid hydrocarbons, the oxidation process in automobile catalytic converters, and the degradation of platinum electrodes in hydrogen fuel cells. As carbon monoxide coverage of the platinum crystal surfaces approached 100-percent, the terraces began to widen - the result of increasing lateral repulsion between the molecules. When the surface pressure reached one torr, the terraces fractured into nanometer-sized clusters. The terraces were re-formed upon removal of the carbon monoxide gas.
"Our observations of the large-scale surface restructuring of stepped platinum highlights the strong connection between coverage of reactant molecules and the atomic structure of the catalyst surface," says Somorjai. "The ability to observe catalytic surfaces at the atomic and molecular levels under actual reaction conditions is the only way such a phenomenon could be detected."
Catalysts - substances that speed up the rates of chemical reactions without themselves being chemically changed - are used to initiate virtually every industrial manufacturing process that involves chemistry. Metal catalysts are the workhorses with platinum being one of the best. Industrial catalysts typically operate under pressures ranging from millitorr to atmospheres, and at temperatures ranging from room to hundreds of degrees Celsius. However, surface science experiments have traditionally been performed under high vacuum conditions and low temperatures.
"Such conditions will likely inhibit any surface restructuring process that requires the overcoming of even moderate activation barriers," Somorjai says.
Says Salmeron, "The unanswered question today is what are the geometry and location of the catalyst atoms when the surfaces are covered with dense layers of molecules, as occurs during a chemical reaction."
Somorjai and Salmeron have for many years been collaborating on the development of instrumentation and techniques that enable them to do catalysis studies under realistic conditions. They now have at their disposal unique high-pressure scanning tunneling microscopes (STM) and an ambient pressure x-ray photoelectron spectroscopy (AP-XPS) beamline operating at the Berkeley Lab's Advanced Light Source, a premier source of synchrotron radiation for scientific research.
"With these two resources, we can image the atomic structure and identify the chemical state of catalyst atoms and adsorbed reactant molecules under industrial-type pressures and temperatures," Salmeron says.
STM images revealed the formation of nanoclusters on the platinum crystal surfaces, and the AP-XPS spectra revealed a change in carbon monoxide electron binding energies. A subsequent collaboration with Lin-Wang Wang, a theorist in Berkeley Lab's Computational Sciences Division, explained the change in structure as the result of the relaxation of the strong repulsion between carbon monoxide molecules that arises from their very high density on the surface when in equilibrium with elevated pressures of the gas.
"In the future, the use of these stable platinum nanoclusters as fuel cell catalysts may help to boost performance and reduce costs," Somorjai says.The next step for Somorjai and Salmeron and their research team will be to determine whether other adsorbed reactants, such as oxygen or hydrogen, also result in the creation of nanoclusters in platinum. They also want to know if nanoclusters can be induced in other metal catalysts as well, such as palladium, silver, copper, rhodium, iron and cobalt.
"If this nanoclustering is a general phenomenon, it will have major consequences for the type of structures that catalysts must have under high-pressure, high-temperature catalytic reaction conditions," Somorjai says.
A paper on this research appears in the journal Science, titled "Break-Up of Stepped Platinum Catalyst Surfaces by High CO Coverage." Co-authoring this paper with Somorjai and Salmeron were Feng Tao, Sefa Dag, Lin-Wang Wang, Zhi Liu, Derek Butcher and Hendrik Bluhm.
This research was supported by the Basic Energy Sciences programs of the DOE Office of Science.
Additional Information
For more information about the research of Gabor Somorjai, visit the Website at
www.cchem.berkeley.edu/gasgrp/
For more information about the research of Miquel Salmeron visit the Website at
stm.lbl.gov/Salmeron_group/home.html
####
About Lawrence Berkeley National Laboratory
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research for DOE’s Office of Science and is managed by the University of California. Visit our Website at www.lbl.gov/
For more information, please click here
Contacts:
Lynn Yarris
(510) 486-5375
Copyright © Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Fuel Cells
![]() Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
Deciphering local microstrain-induced optimization of asymmetric Fe single atomic sites for efficient oxygen reduction August 8th, 2025
![]() Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
Current and Future Developments in Nanomaterials and Carbon Nanotubes: Applications of Nanomaterials in Energy Storage and Electronics October 28th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








