Home > Press > Livermore researchers use carbon nanotubes for molecular transport
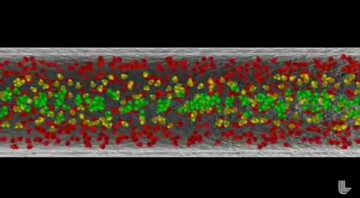 |
| FAST FLOW THROUGH CARBON NANOTUBES: The animation starts with the depiction of the water flow through a regular "rough" pipe. The molecules near the wall stick to it and move much slower than the molecules in the middle of the pipe. Colors indicate the speed of the molecules -- green are fast, yellow are slower, red are the slowest.
The rough pipe fades and the carbon nanotube appears. All the molecules in the carbon nanotube move fast (green). They do not stick to the surface of the nanotube because that surface is very slippery. The water molecules travel in chains because they interact with each other strongly via hydrogen bonds. These two effects (the slippery nanotube surface and formation of water molecule chains inside the nanotube) combine to produce this phenomenon of ultra-fast flow through carbon nanotubes. Animation by Kwei-Yu Chu/LLNL - https://publicaffairs.llnl.gov/news/news_releases/2008/NR-08-06-03.html |
Abstract:
Molecular transport across cellular membranes is essential to many of life's processes, for example electrical signaling in nerves, muscles and synapses.
In biological systems, the membranes often contain a slippery inner surface with selective filter regions made up of specialized protein channels of sub-nanometer size. These pores regulate cellular traffic, allowing some of the smallest molecules in the world to traverse the membrane extremely quickly, while at the same time rejecting other small molecules and ions.
Livermore researchers use carbon nanotubes for molecular transport
LIVERMORE, CA | Posted on June 9th, 2008Researchers at Lawrence Livermore National Laboratory are mimicking that process with manmade carbon nanotube membranes, which have pores that are 100,000 times smaller than a human hair, and were able to determine the rejection mechanism within the pores.
"Hydrophobic, narrow diameter carbon nanotubes can provide a simplified model of membrane channels by reproducing these critical features in a simpler and more robust platform," said Olgica Bakajin, who led the LLNL team whose study appeared in the June 6 online edition of the journal Proceedings of the National Academy of Sciences.
In the initial discovery, reported in the May 19, 2006 issue of the journal Science, the LLNL team found that water molecules in a carbon nanotube move fast and do not stick to the nanotube's super smooth surface, much like water moves through biological channels. The water molecules travel in chains - because they interact with each other strongly via hydrogen bonds.
"You can visualize it as mini-freight trains of chain-bonded water molecules flying at high speed through a narrow nanotube tunnel," said Hyung Gyu Park, an LLNL postdoctoral researcher and a team member.
One of the most promising applications for carbon nanotube membranes is sea water desalination. These membranes will some day be able to replace conventional membranes and greatly reduce energy use for desalination.
In the recent study, the researchers wanted to find out if the membranes with 1.6 nanometer (nm) pores reject ions that make up common salts. In fact, the pores did reject the ions and the team was able to understand the rejection mechanism.
"Our study showed that pores with a diameter of 1.6nm on the average, the salts get rejected due to the charge at the ends of the carbon nanotubes," said Francesco Fornasiero, an LLNL postdoctoral researcher, team member and the study's first author
Fast flow through carbon nanotube pores makes nanotube membranes more permeable than other membranes with the same pore sizes. Yet, just like conventional membranes, nanotube membranes exclude ions and other particles due to a combination of small pore size and pore charge effects.
"While carbon nanotube membranes can achieve similar rejection as membranes with similarly sized pores, they will provide considerably higher permeability, which makes them potentially much more efficient than the current generation of membranes," said Aleksandr Noy, a senior member of the LLNL team.
Researchers will be able to build better membranes when they can independently change pore diameter, charge and material that fills gaps between carbon nanotubes.
####
About Lawrence Livermore National Laboratory
Founded in 1952, Lawrence Livermore National Laboratory is a national security laboratory, with a mission to ensure national security and apply science and technology to the important issues of our time. Lawrence Livermore National Laboratory is managed by Lawrence Livermore National Security, LLC for the U.S. Department of Energy's National Nuclear Security Administration.
For more information, please click here
Contacts:
Anne M. Stark
(925) 422-9799
Copyright © Lawrence Livermore National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Videos/Movies
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
New X-ray imaging technique to study the transient phases of quantum materials December 29th, 2022
![]() Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
Solvent study solves solar cell durability puzzle: Rice-led project could make perovskite cells ready for prime time September 23rd, 2022
![]() Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Scientists prepare for the world’s smallest race: Nanocar Race II March 18th, 2022
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings/Nanosheets
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Water
![]() Taking salt out of the water equation October 7th, 2022
Taking salt out of the water equation October 7th, 2022
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








