Home > Press > Warming up for Magnetic Resonance Imaging: Higher temperatures yield tunable, supersensitive Hyper-CEST MRI
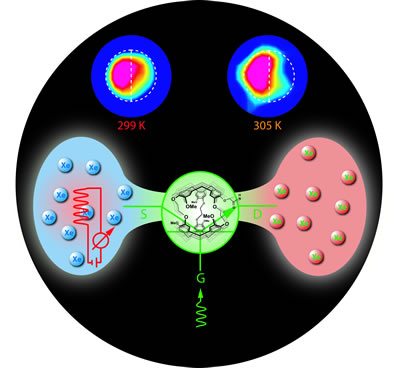 |
| In the technique known as "temperature-controlled molecular depolarization gates," an atom of hyperpolarized xenon from the pool at left enters a cryptophane cage, center, which is part of a biosensor attached to a specific molecular target. A burst of tuned rf energy depolarizes the xenon, which is then ejected back into the pool by chemical exchange with the next incoming xenon atom. Depolarized xenon (right) stands out in the larger hyperpolarized pool and thus enhances the contrast of the nearby target molecule. At top, a phantom half-filled with agarose beads, to which biosensors are attached, shows how image contrast can be enhanced and controlled by temperature: a 6-K temperature increase quickly depolarizes the xenon in the vicinity of the target beads. |
Abstract:
Standard magnetic resonance imaging, MRI, is a superb diagnostic tool but one that suffers from low sensitivity, requiring patients to remain motionless for long periods of time inside noisy, claustrophobic machines. A promising new MRI method, much faster, more selective — able to distinguish even among specific target molecules — and many thousands of times more sensitive, has now been developed in the laboratory by researchers at the Department of Energy's Lawrence Berkeley National Laboratory and the University of California at Berkeley.
Warming up for Magnetic Resonance Imaging: Higher temperatures yield tunable, supersensitive Hyper-CEST MRI
Berkeley, CA | Posted on May 10th, 2008The key to the new technique is called "temperature-controlled molecular depolarization gates." It builds on a series of previous developments in MRI and the closely related field of nuclear magnetic resonance, NMR (which instead of an image yields a spectrum of molecular information), by members of the laboratories of Alexander Pines and David Wemmer at Berkeley Lab and UC Berkeley. Pines is the Glenn T. Seaborg Professor of Chemistry at the University of California at Berkeley and a senior scientist in Berkeley Lab's Materials Sciences Division. Wemmer is Professor of Chemistry at UC Berkeley and a member of Berkeley Lab's Physical Biosciences Division.
The technique was developed by a team of past and present Pines and Wemmer lab members headed by Leif Schröder of Berkeley Lab's Materials Sciences Division and including Lana Chavez, Tyler Meldrum, Monica Smith, and Thomas Lowery; the researchers outline their results in the international edition of the journal Angewandte Chemie.
"The new method holds the promise of combining a set of proven NMR tools for the first time into a practical, supersensitive diagnostic system for imaging the distribution of specific molecules on such targets as tumors in human subjects," says lead author Schröder, "or even on individual cancer cells."
Laying the groundwork: hyperpolarization and cryptophane biosensors lead to Hyper-CEST MRI
MRI and NMR make use of the quantum-mechanical phenomenon known as nuclear spin; nuclei with odd numbers of protons or neutrons have net magnetic moment and will orient themselves like tiny bar magnets, spin "up" or spin "down," in a strong magnetic field. If the spinning nuclei are knocked off-axis by a jolt of radio-frequency (rf) energy, they wobble or precess at a characteristic rate, a rate that is strongly conditioned by their immediate chemical neighbors. During a certain relaxation time (typical of each atomic species in a specific environment), the nuclei reorient themselves and emit a radio signal that reveals both their position and their chemical surroundings.
The spin-up state requires fractionally less energy, so there's typically a slight excess of spin-up nuclei, about one in a hundred thousand (.001 percent), and it's this tiny difference that yields a useful signal. In clinical settings MRI is usually done using hydrogen nuclei, protons, which are ubiquitous in the human body. But other nuclear species, notably the noble gas xenon, offer advantages over hydrogen that in the case of xenon include a virtual absence of background signal, since there is no xenon in biological systems.
Xenon is particularly useful in MRI and NMR because the spins of its nuclei are readily polarized, in a process involving contact with rubidium vapor irradiated with a laser beam. In such "hyperpolarized" xenon, the excess of spin-up nuclei can be as much as 20 percent, which gives a far stronger signal than hydrogen's .001 percent spin-up excess. Moreover, hyperpolarized xenon has a much longer relaxation time than hydrogen.
Now add the ability to associate a single xenon nucleus with a specific molecular target, for example a protein or sugar on the surface of a cancer cell. To do this, the Pines and Wemmer labs have created biosensors equipped with cages that take up and hold onto xenon atoms; the cages, molecules called cryptophanes, are linked to ligands that target specific molecules of interest. Xenon biosensors engineered with several different ligands can be used at the same time; once in place, biosensors carrying hyperpolarized xenon can localize the MRI signals from a range of different molecules on the target.
The final advance underlying the new technique is called Hyper-CEST: hyperpolarized xenon chemical-exchange saturation transfer. While biosensors can bring the xenon to specific molecular targets, in realistic applications relatively few of these are present, only about one percent compared to the total amount of free xenon injected near that target. The signal from the polarized xenon inside the biosensor cages is consequently much fainter than that from the uncaged polarized xenon nearby.
"About 60 percent of the biosensor cages are filled with xenon," says Schröder, "but the problem is, you get only a tiny, broad NMR signal from the xenon when it is inside the cage. On the other hand, you have thousands of xenon nuclei just sitting around the cage."
The trick then is to depolarize the xenon nuclei in the immediate vicinity of the cages, which will serve to outline the target in high contrast against the surrounding hyperpolarized xenon pool. This is done through chemical exchange, as xenon atoms are constantly entering and leaving the biosensor cages.
A polarized xenon atom from the pool enters the cryptophane cage, which alters the xenon's resonance frequency, allowing it to be depolarized by rf radiation tuned to a specific frequency. The depolarized xenon atom is then exchanged for a new, incoming polarized atom and reenters the pool. In this way the buildup of nearby depolarized nuclei quickly outlines the target.
Because it produces a much stronger signal, Hyper-CEST acquires images thousands of times faster than would imaging the caged xenon directly. Yet it retains the great advantages of cryptophane biosensors, including their ability to "multiplex," or detect different targets at the same time.
"Slight differences in cage composition, involving only a carbon atom or two, affect the frequency of the signal from the xenon and produce distinct peaks in the NMR spectrum," says team member Tyler Meldrum, of the Materials Sciences Division. "If we design different cages for different xenon frequencies, we can put them all in at once and, by selectively tuning the rf pulses, see peaks at the frequencies corresponding to each kind of cage."
The final step
The processes described above — hyperpolarizing the xenon, caging it in biosensors, and building up depolarized xenon in the immediate vicinity of the target through chemical exchange and selective bursts of rf radiation — led to the development of Hyper-CEST MRI. But until now, Hyper-CEST MRI has only been tested at room temperature.
Using biosensor cages as temperature-controlled molecular depolarization gates makes Hyper-CEST MRI possible at a range of higher-than-room temperatures. Because the technique regulates the exchange rate of hyperpolarized-to-depolarized nuclei through the cages, biosensors regulated this way have been nicknamed "transpletors," by analogy to the transistors that act as gates for the flow of electrons from source to drain in electronic systems.
Hyper-CEST at a range of temperatures has many advantages. Most basic is that biomedical MRI must operate at body temperature. Aside from this practical consideration, temperature determines the rates at which different kinds of cryptophane-cage hosts react with their xenon-atom guests. And increasing temperature dramatically increases chemical exchange rates.
"At room temperature, a xenon atom will stay approximately 50 milliseconds inside the cage before it leaves again," says team member Monica Smith, of Berkeley Lab's Physical Biosciences Division. "Approaching body temperature, the time inside the cage decreases by at least factor of 10."
The ability to achieve high-contrast images, multiplexed to identify a range of molecular targets, and to do so in a short time, offers many benefits to patients and physicians.
"Doctors attempting to characterize tumors very often have to take biopsies, and that's painful for the patient, so they usually prefer to take only one biopsy," says Schröder. "But then they have to run all their tests on this very little tissue. So they would be happy with a method where you have a toolbox of sensors, you throw them all in and wait to let them bind, and then do your tests at the different frequencies and you see what sensors are present, detecting the different proteins. We showed that the exchange rate is so high at increased temperature that you can use a very selective rf pulse."
Enabling fast, sensitive, molecule-specific NMR and MRI in humans and other living subjects is perhaps the most evident advantage of the new technique, but possible applications don't end there. For example, the method offers a better way to study chemical exchange in nanostructures like zeolites, which are important in catalysis, or in versatile carbon nanotubes. Temperature-controlled depolarization is a breakthrough for NMR and MRI that will find uses in a variety of fields.
"Temperature-controlled molecular depolarization gates in nuclear magnetic resonance," by Leif Schröder, Lana Chavez, Tyler Meldrum, Monica Smith, Thomas J. Lowery, David E. Wemmer, and Alexander Pines, will soon appear in the international edition of Angewandte Chemie and is available online to subscribers at http://dx.doi.org/10.1002/anie.200800382.
This research was supported by the Department of Energy's Office of Science, Office of Basic Energy Sciences; by the Deutsche Forschungsgemeinschaft; and by the University of California's Biotechnology Research and Education Program.
####
About Lawrence Berkeley National Laboratory
Berkeley Lab is a U.S. Department of Energy national laboratory located in Berkeley, California. It conducts unclassified scientific research and is managed by the University of California.
For more information, please click here
Contacts:
Media Contact:
Paul Preuss
(510) 486-6249
Research Contact:
Alyse Jacobson
(510) 486-6097
Copyright © Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings/Nanosheets
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








