Home > Press > Luna Innovations Successfully Demonstrates MRI Contrast Agent
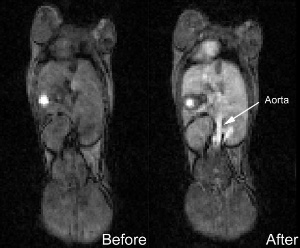 |
| Luna Innovations has produced and demonstrated a prototype contrast imaging agent designed to be more effective at enhancing images and is potentially safer than current MRI agents. Here, images of a mouse show contrast quality before and 30 minutes after administration with noticeable enhancement. Luna is building a portfolio of nanomedicines aimed at disease targeting and diagnostic imaging based on their new HYDROCHALARONE(TM) family of molecules. (Photo:Business Wire) |
Abstract:
Nanomedicine Candidate Could Improve Quality and Safety in MRI Procedures
Luna Innovations Successfully Demonstrates MRI Contrast Agent
ROANOKE, VA | Posted on April 30th, 2008 The product development team at the nanoWorks division of Luna Innovations Incorporated (NASDAQ:LUNA) has produced a novel class of molecules designed to provide a new approach to improve the quality of magnetic resonance imaging (MRI). Luna's molecules have initially proven to be more effective at enhancing images and are potentially safer than current MRI agents. This new class of molecules provides a platform upon which Luna plans to build a portfolio of nanomedicines aimed at disease targeting and diagnostic imaging.
MRI is a non-invasive diagnostic procedure that enables physicians to visualize organs, soft tissues, bone and virtually all other internal body structures through magnetic interactions. MRI does not use ionizing radiation (x-rays). These medical imaging procedures are enhanced by the use of contrast agents to further improve diagnosis. The MRI contrast agent market is reported to be more than $1 billion annually and is currently dominated by gadolinium chelate-based agents which are the subject of class-action lawsuit concerns due to the potential release of gadolinium into the body.
The new class of molecules discovered by Luna is called HYDROCHALARONETM [ hī-drō-kal-a-rōne ]— Hydro, meaning water, combined with Chalaro, the Greek word for relax. The level of relaxivity is the characteristic of molecules that provides the image enhancement. "The high relaxivity in Hydrochalarone means fewer molecules are needed to obtain a better quality image," said Robert Lenk, President of Luna's nanoWorks division. "Our studies demonstrate that our proprietary nanomaterials do not release gadolinium under conditions which are found in the human body. Our imaging studies in mice have shown Hydrochalarone improves image quality up to 30 minutes after injection at a dose 20 times lower than that used with current agents."
"Achieving high magnetic resonance relaxivity with a small, biologically inert, chemical moiety that can be derivatized for targeted tissue delivery, cell tracking, or inclusion as part of a nanoparticle drug delivery vehicle is a Holy Grail within the fast evolving field of biomarker development," said Dr. Joseph Ackerman renowned MRI researcher and Chemistry Department Chairman at Washington University in St. Louis. "The design and production of Hydrochalarones by scientists at Luna nanoWorks may herald such an advance."
Luna's HYDROCHALARONETM was selected for preclinical studies and a collaboration with National Cancer Institute's Nanotechnology Characterization Laboratory (NCL). "We hope within 12 months the NCL will provide us a complete preclinical package which will contribute to an Investigational New Drug application," said Lenk. "The end goal of Luna's product development effort with the Hydrochalarone is using it as a fundamental building block that will generate a portfolio of novel imaging agents targeted to reveal diagnostic information specific for a variety of different diseases, such as cancer tumors, sites of inflammation and plaque related to coronary artery diseases, as announced in our previous press release."
"We are continuing to develop innovative new diagnostics and therapeutics based on our proprietary nanotechnologies which have the potential to change medical practice," stated Kent Murphy, Luna's Chairman and CEO. "According to the Food and Drug Administration, the use of today's gadolinium chelate-based contrast agents may pose a significant health threat to patients, particularly in those with kidney or liver disease. With Luna's new molecule, we anticipate the development of a portfolio of products based on a new approach to enhancing medical images and provide a better outcome for patients."
####
About Luna Innovations Incorporated
Luna Innovations Incorporated (www.lunainnovations.com) develops and manufactures new-generation products for the healthcare, telecommunications, energy and defense markets. Luna’s products are used to measure, monitor, protect and improve critical processes in the markets we serve. Luna nanoWorks is a division of Luna Innovations. With a world-class nanomaterial manufacturing facility in Danville, Virginia, the nanoWorks Division is developing products empowered by nanomaterials with applications in diagnostics, therapeutics, and organic solar cells.
Forward Looking / Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995:
This press release includes information that constitutes “forward-looking statements” made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995, including but not limited to Luna Innovations’ and its nanoWorks division’s expected development of potential products with respect to MRI contrast agents. The company attempts, whenever possible, to identify forward-looking statements by words such as “intends,” “will,” “plans,” “anticipates,” “expects,” “may,” “estimates,” “believes,” “should,” “projects,” or “continue,” or the negative of those words and other comparable words. Similarly, statements that describe the company’s business strategy, goals, prospects, opportunities, outlook, objectives, plans or intentions are also forward-looking statements. Actual events or results may differ materially from the expectations expressed in such forward-looking statements as a result of various factors, including risks and uncertainties, many of which are beyond the company’s control. Factors that could cause actual results to differ materially from the expectations expressed in such forward-looking statements include, but are not limited to: the company’s ability to successfully identify market needs for new products; the potential adverse effects of nanotechnology, nanomedicines, or imaging agents whether real or perceived; and the potential limitations of regulatory requirements in obtaining clearance by the U.S. Food and Drug Administration or other regulatory agencies for the company’s products. Additional factors that may affect the future results of the company are set forth in the company’s quarterly and annual reports on Form 10-Q and Form 10-K, respectively, and other filings with the Securities and Exchange Commission (“SEC”), which are available at the SEC’s website at http://www.sec.gov, and at the Company’s website at http://www.lunainnovations.com. These risk factors are updated from time to time through the filing of periodic reports with the SEC. The statements made in this press release are based on information available to the company as of the date of this release and Luna Innovations undertakes no obligation to update any of the forward-looking statements herein after the date of this press release.
For more information, please click here
Contacts:
Media Contact:
Luna Innovations Incorporated
Karin Clark
1-540-769-8400
Email:
or
Investor Contact:
Qorvis Communications
Sally Beerbower
1-703-744-7800
Email:
Copyright © Business Wire 2008
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








