Home > Press > Cell Division Studies May Hold Keys to Cancer Treatments
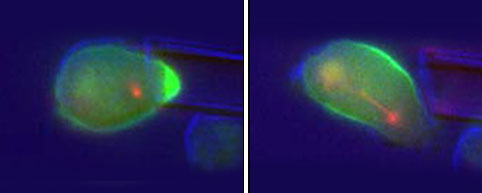 |
| Left: Contractile proteins (bright green) accumulate where micropipette deforms cell shape. Right: Contractile proteins redistribute along cell’s midsection to drive division; mitotic spindle—microtubules apparent in early stages of cytokinesis—shown in red. Credit: Robinson Lab / JHU |
Abstract:
Through a comprehensive investigation of the fundamental process of cell division, Doug Robinson— assistant professor in the Department of Cell Biology at the Johns Hopkins School of Medicine and Institute for NanoBioTechnology affiliated faculty member—hopes to spur interest in some novel approaches to cancer treatment and prevention.
Cell Division Studies May Hold Keys to Cancer Treatments
Baltimore, MD | Posted on January 29th, 2008To keep tissues and organs healthy, cells must divide. Robinson explains that each day, billions of cells are replaced in the human body through the process of reshaping one cell into two—or cytokinesis. "More than 100 million cytokinetic events are going on in the human body at any given moment," he says. Interference with this process can result in cytokinetic failure and ultimately, sickness and disease.
"On the one hand, cells appear to be very passive, and on the other hand, they are able to completely and dynamically remodel," he says. "At the interface of these two different fundamental properties, the cell does what it needs to do to survive. Cytokinetic failure is an example of where a very regulated and organized shape change process fails. This has major implications for how the cell protects its genome and future cell cycles."
To quickly and inexpensively study many aspects of cell division, Robinson's lab uses the protozoan Dictyostelium discoideum, which exhibits features of mammalian cells during cytokinesis.
"My lab aims to understand how cell division happens —from the size-scale of entire organisms down to the nanoscale actions and interactions of individual proteins and molecules," Robinson says. To that end, his lab takes a three-pronged approach. First, they study the seven to 10 proteins (such as actin, myosin-II and dynacortin) involved in the physical contractions the cell must perform to successfully divide. Second, they want to identify other components of the pathways regulating cytokinesis. And third, they'd like to discover new small molecule inhibitors of cell division. Results from this last avenue of investigation—the pharmacological research focus— he hopes, will lead to new tools for studying cell division and with some luck, might even lead to new drug treatments for diseases such as cancer.
Several members of Robinson's team are observing what happens when cells are physically assaulted by a very small force from a micropipette. They have found that cells recover from these tiny deformations by directing critical proteins through a quick-reacting control system. This work is being done in collaboration with Pablo Iglesias, professor in the Whiting School of Engineering's Department of Electrical and Computer Engineering, who is also an INBT affiliated faculty member.
Other experiments investigating cytokinesis involve inhibiting or modifying the gene products at the molecular level that interfere with the cell division process. Genetic approaches include knockouts, RNA interference, and suppression analysis, among others.
But Robinson does not plan to stop there. To study the role of the cytoskeleton (the cellular scaffolding that gives a cell its shape) in cytokinesis, he says he and several collaborators are working on ways to reduce the system down to its most elemental components.
"We would like to build a device that can do single molecule detection in a purified cytoskeletal network and allow us to mechanically perturb the network while simultaneously observing the behavior of the individual proteins," he says. "If we can make a biomimetic system (a man-made procedure designed to mimic a natural process) that matches exactly what we see in a dividing cell, we can then study this reconstituted system and hopefully begin to understand how cytokinesis actually works."
To read more about Doug Robinson's research, go to
# http://esgweb1.nts.jhu.edu/cellbio/robinson/index.html
To watch movies of cytokinesis, go to
# http://esgweb1.nts.jhu.edu/cellbio/robinson/movies.html
Glossary:
Knockout: having a single gene removed from the genome
RNA interference (RNAi): using double-stranded RNA to inhibit gene expression.
Suppression analysis: identifies genes that are functionally related to another gene.
####
About Institute for NanoBioTechnology
The Institute for NanoBioTechnology at Johns Hopkins University is revolutionizing health care by bringing together internationally renowned expertise in medicine, engineering, the sciences, and public health to create new knowledge and groundbreaking technologies.
INBT programs in research, education, outreach, and technology transfer are designed to foster the next wave of nanobiotechnology innovation.
Approximately 150 faculty are affiliated with INBT and are also members of the following Johns Hopkins institutions: Krieger School of Arts and Sciences, Whiting School of Engineering, School of Medicine, Bloomberg School of Public Health, and Applied Physics Laboratory.
For more information, please click here
Contacts:
Mary Spiro
Copyright © Institute for NanoBioTechnology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








