Home > Press > Old Molecule, New Tricks: Chemistry professors develop an electrochemical method for extracting uranium, and potentially other metal ions, from solution
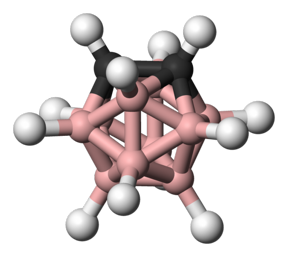 |
| An ortho-carborane, which is a three-dimensional cluster molecule composed of boron, carbon and hydrogen atoms Photo Credit: WIKIMEDIA COMMONS |
Abstract:
Fifty years ago, scientists hit upon what they thought could be the next rocket fuel. Carboranes — molecules composed of boron, carbon and hydrogen atoms clustered together in three-dimensional shapes — were seen as the possible basis for next-generation propellants due to their ability to release massive amounts of energy when burned.
Old Molecule, New Tricks: Chemistry professors develop an electrochemical method for extracting uranium, and potentially other metal ions, from solution
Santa Barbara, CA | Posted on January 24th, 2020It was technology that at the time had the potential to augment or even surpass traditional hydrocarbon rocket fuel, and was the subject of heavy investment in the 1950s and 60s.
But things didn’t pan out as expected.
“It turns out that when you burn these things you actually form a lot of sediment,” said Gabriel Ménard, an assistant professor in UC Santa Barbara’s Department of Chemistry and Biochemistry. In addition to other problems found when burning this so-called “zip fuel,” its residue also gummed up the works in rocket engines, and so the project was scrapped.
“So they made these huge stockpiles of these compounds, but they actually never used them,” Ménard said.
Fast forward to today, and these compounds have come back into vogue with a wide range of applications, from medicine to nanoscale engineering. For Ménard and fellow UCSB chemistry professor Trevor Hayton, as well as Tel Aviv University chemistry professor Roman Dobrovetsky, carboranes could hold the key to more efficient uranium ion extraction. And that, in turn, could enable things like better nuclear waste reprocessing and uranium (and other metal) recovery from seawater.
Their research — the first example of applying electrochemical carborane processes to uranium extraction — is published in a paper that appears in the journal Nature.
Key to this technology is the versatility of the cluster molecule. Depending on their compositions these structures can resemble closed cages, or more open nests, due to control of the compound’s redox activity — its readiness to donate or gain electrons. This allows for the controlled capture and release of metal ions, which in this study was applied to uranium ions.
“The big advancement here is this ‘catch and release’ strategy where you can switch between two states, where one state binds the metal and another state releases the metal,” Hayton said.
Conventional processes, such as the popular PUREX process that extracts plutonium and uranium, rely heavily on solvents, extractants and extensive processing.
“Basically, you could say it’s wasteful,” Ménard said. “In our case, we can do this electrochemically — we can capture and release the uranium with the flip of a switch.
“What actually happens,” added Ménard, “is that the cage opens up.” Specifically, the formerly closed ortho-carborane becomes an opened nido- (“nest”) carborane capable of capturing the positively-charged uranium ion.
Conventionally, the controlled release of extracted uranium ions, however, is not as straightforward and can be somewhat messy. According to the researchers, such methods are “less established and can be difficult, expensive and or destructive to the initial material.”
But here, the researchers have devised a way to reliably and efficiently flip back and forth between open and closed carboranes, using electricity. By applying an electrical potential using an electrode dipped in the organic portion of a biphasic system, the carboranes can receive and donate the electrons needed to open and close and capture and release uranium, respectively.
“Basically you can open it up, capture uranium, close it back up and then release uranium,” Ménard said. The molecules can be used multiple times, he added.
This technology could be used for several applications that require the extraction of uranium and by extension, other metal ions. One area is nuclear reprocessing, in which uranium and other radioactive “trans-uranium” elements are extracted from spent nuclear material for storage and reuse (the PUREX process).
“The problem is that these trans-uranium elements are very radioactive and we need to be able to store these for a very long time because they’re basically very dangerous,” Ménard said. This electrochemical method could allow for the separation of uranium from plutonium, similar to the PUREX process, he explained. The extracted uranium could then be enriched and put back into the reactor; the other high-level waste can be transmuted to reduce their radioactivity.
Additionally, the electrochemical process could also be applied to uranium extraction from seawater, which would ease pressure on the terrestrial mines where all uranium is currently sourced.
“There’s about a thousand times more dissolved uranium in the oceans than there are in all the land mines,” Ménard said. Similarly, lithium — another valuable metal that exists in large reserves in seawater — could be extracted this way, and the researchers plan to take this research direction in the near future.
“This gives us another tool in the toolbox for manipulating metal ions and processing nuclear waste or doing metal capture out of oceans,” Hayton said. “It’s a new strategy and new method to achieve these types of transformations.”
Research in this study was conducted also by Megan Keener (lead author), Camden Hunt and Timothy G. Carroll at UCSB; and by Vladimir Kampel at Tel Aviv University.
####
For more information, please click here
Contacts:
Sonia Fernandez
(805) 893-4765
Shelly Leachman
(805) 893-8726
Copyright © University of California, Santa Barbara
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








