Home > Press > Tweaking electrolyte makes better lithium-metal batteries: A pinch of electrolyte additive gives rechargeable battery stability, longer life
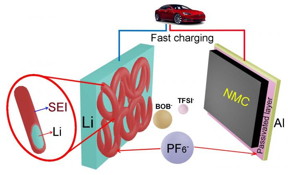 |
| This is an artist's illustration shows how PNNL's addition of the chemical lithium hexafluorophosphate to a dual-salt, carbonate solvent-based electrolyte makes rechargeable lithium-metal batteries stable, charge quickly, have a high voltage, and go longer in between charges. CREDIT Pacific Northwest National Laboratory |
Abstract:
Scientists have found adding a pinch of something new to a battery's electrolyte gives the energy storage devices more juice per charge than today's commonly used rechargeable batteries.
Tweaking electrolyte makes better lithium-metal batteries: A pinch of electrolyte additive gives rechargeable battery stability, longer life
Richland, WA | Posted on March 2nd, 2017New, early-stage research shows adding a small amount of the chemical lithium hexafluorophosphate to a dual-salt, carbonate solvent-based electrolyte can make rechargeable lithium-metal batteries stable, charge quickly and have a high voltage.
"A good lithium-metal battery will have the same lifespan as the lithium-ion batteries that power today's electric cars and consumer electric devices, but also store more energy so we can drive longer in between charges," said chemist Wu Xu of the Department of Energy's Pacific Northwest National Laboratory. Xu is a corresponding author on a paper published today in the journal Nature Energy.
Battery basics
Most of the rechargeable batteries used today are lithium-ion batteries, which have two electrodes: one that's positively charged and contains lithium, and another negative one that's typically made of graphite. Electricity is generated when electrons flow through a wire that connects the two.
To control the electrons, positively charged lithium atoms shuttle from one electrode to the other through another path, the electrolyte solution in which the electrodes sit. But graphite can't store much energy, limiting the amount of energy a lithium-ion battery can provide smart phones and electric vehicles.
When lithium-based rechargeable batteries were first developed in the 1970s, researchers used lithium metal for the negative electrode, called an anode. Lithium was chosen because it has ten times more energy storage capacity than graphite. Problem was, the lithium-carrying electrolyte reacted with the lithium anode. This caused microscopic lithium nanoparticles and branches called dendrites to grow on the anode surface, and led the early batteries to fail.
Many have tweaked rechargeable batteries over the years in an attempt to resolve the dendrite problem. Researchers switched to other materials such as graphite for the anode. Scientists have also coated anodes with protective layers, while others have created electrolyte additives. Some solutions eliminated dendrites but also resulted in impractical batteries with little power. Other methods only slowed, but didn't stop, dendrite growth.
Next-generation storage
Thinking today's rechargeable lithium-ion batteries with graphite anodes could be near their peak energy capacity, PNNL is taking another look at the older design with lithium metal as an anode. Xu and colleagues were part of earlier PNNL research seeking a better-performing electrolyte. The electrolytes they tried produced either a battery that didn't have problematic dendrites and was super-efficient but charged very slowly and couldn't work in higher-voltage batteries, or a faster-charging battery that was unstable and had low voltages.
Next, they tried adding small amounts of a salt that's already used in lithium-ion batteries, lithium hexafluorophosphate, to their fast-charging electrolyte. They paired the newly juiced-up electrolyte with a lithium anode and a lithium nickel manganese cobalt oxide cathode. It turned out to be a winning combination, resulting in a fast, efficient, high-voltage battery.
The additive enabled a 4.3-volt battery that retained more than 97 percent of its initial charge after 500 repeated charges and discharges, while carrying 1.75 milliAmps of electrical current per square centimeter of area. It took the battery about one hour to fully charge.
Inexpensive protection
The battery performed well largely because the additive helps create a robust protective layer of carbonate polymers on the battery's lithium anode. This thin layer prevents lithium from being used up in unwanted side reactions, which can kill a battery.
And, because the additive is already an established component of lithium-ion batteries, it's readily available and relatively inexpensive. The small amounts needed - just 0. 6 percent of the electrolyte by weight - should also further lower the electrolyte's cost.
Xu and his team continue to evaluate several ways to make rechargeable lithium-metal batteries viable, including improving electrodes, separators and electrolytes. Specific next steps include making and testing larger quantities of their electrolyte, further improving the efficiency and capacity retention of a lithium-metal battery using their electrolyte, increasing material loading on the cathode and trying a thinner anode.
###
This research was supported by the Department of Energy's Office of Energy Efficiency and Renewable Energy. Researchers performed microscopy and spectroscopy characterizations of battery materials at EMSL, the Environmental Molecular Sciences Laboratory, a DOE Office of Science national User Facility at PNNL. The battery electrodes were made at DOE's Cell Analysis, Modeling, and Prototyping Facility at Argonne National Laboratory.
####
About Pacific Northwest National Laboratory
EMSL, the Environmental Molecular Sciences Laboratory, is a DOE Office of Science User Facility. Located at Pacific Northwest National Laboratory in Richland, Wash., EMSL offers an open, collaborative environment for scientific discovery to researchers around the world. Its integrated computational and experimental resources enable researchers to realize important scientific insights and create new technologies. Follow EMSL on Facebook, LinkedIn and Twitter.
Interdisciplinary teams at Pacific Northwest National Laboratory address many of America's most pressing issues in energy, the environment and national security through advances in basic and applied science. Founded in 1965, PNNL employs 4,400 staff and has an annual budget of nearly $1 billion. It is managed by Battelle for the U.S. Department of Energy's Office of Science. As the single largest supporter of basic research in the physical sciences in the United States, the Office of Science is working to address some of the most pressing challenges of our time. For more information on PNNL, visit the PNNL News Center, or follow PNNL on Facebook, Google+, Instagram, LinkedIn and Twitter.
For more information, please click here
Contacts:
Franny White
509-375-6904
Copyright © Pacific Northwest National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
![]() A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
A battery’s hopping ions remember where they’ve been: Seen in atomic detail, the seemingly smooth flow of ions through a battery’s electrolyte is surprisingly complicated February 16th, 2024
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Battery Technology/Capacitors/Generators/Piezoelectrics/Thermoelectrics/Energy storage
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








