Home > Press > Targeted drug delivery with these nanoparticles can make medicines more effective: Nanoparticles wrapped inside human platelet membranes serve as new vehicles for targeted drug delivery
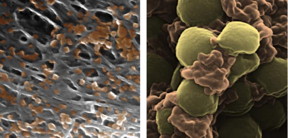 |
| Pseudocolored scanning electron microscope images of platelet-membrane-coated nanoparticles (orange) binding to the lining of a damaged artery (left) and to MRSA bacteria (right). Each nanoparticle is approximately 100 nanometers in diameter, which is one thousand times thinner than an average sheet of paper.
CREDIT: Zhang Research Group, UC San Diego Jacobs School of Engineering. |
Abstract:
Nanoparticles disguised as human platelets could greatly enhance the healing power of drug treatments for cardiovascular disease and systemic bacterial infections. These platelet-mimicking nanoparticles, developed by engineers at the University of California, San Diego, are capable of delivering drugs to targeted sites in the body -- particularly injured blood vessels, as well as organs infected by harmful bacteria. Engineers demonstrated that by delivering the drugs just to the areas where the drugs were needed, these platelet copycats greatly increased the therapeutic effects of drugs that were administered to diseased rats and mice.
Targeted drug delivery with these nanoparticles can make medicines more effective: Nanoparticles wrapped inside human platelet membranes serve as new vehicles for targeted drug delivery
San Diego, CA | Posted on September 17th, 2015The research, led by nanoengineers at the UC San Diego Jacobs School of Engineering, was published online Sept. 16 in Nature.
"This work addresses a major challenge in the field of nanomedicine: targeted drug delivery with nanoparticles," said Liangfang Zhang, a nanoengineering professor at UC San Diego and the senior author of the study. "Because of their targeting ability, platelet-mimicking nanoparticles can directly provide a much higher dose of medication specifically to diseased areas without saturating the entire body with drugs."
The study is an excellent example of using engineering principles and technology to achieve "precision medicine," said Shu Chien, a professor of bioengineering and medicine, director of the Institute of Engineering in Medicine at UC San Diego, and a corresponding author on the study. "While this proof of principle study demonstrates specific delivery of therapeutic agents to treat cardiovascular disease and bacterial infections, it also has broad implications for targeted therapy for other diseases such as cancer and neurological disorders," said Chien.
The ins and outs of the platelet copycats
On the outside, platelet-mimicking nanoparticles are cloaked with human platelet membranes, which enable the nanoparticles to circulate throughout the bloodstream without being attacked by the immune system. The platelet membrane coating has another beneficial feature: it preferentially binds to damaged blood vessels and certain pathogens such as MRSA bacteria, allowing the nanoparticles to deliver and release their drug payloads specifically to these sites in the body.
Enclosed within the platelet membranes are nanoparticle cores made of a biodegradable polymer that can be safely metabolized by the body. The nanoparticles can be packed with many small drug molecules that diffuse out of the polymer core and through the platelet membrane onto their targets.
To make the platelet-membrane-coated nanoparticles, engineers first separated platelets from whole blood samples using a centrifuge. The platelets were then processed to isolate the platelet membranes from the platelet cells. Next, the platelet membranes were broken up into much smaller pieces and fused to the surface of nanoparticle cores. The resulting platelet-membrane-coated nanoparticles are approximately 100 nanometers in diameter, which is one thousand times thinner than an average sheet of paper.
This cloaking technology is based on the strategy that Zhang's research group had developed to cloak nanoparticles in red blood cell membranes. The researchers previously demonstrated that nanoparticles disguised as red blood cells are capable of removing dangerous pore-forming toxins produced by MRSA, poisonous snake bites and bee stings from the bloodstream.
By using the body's own platelet membranes, the researchers were able to produce platelet mimics that contain the complete set of surface receptors, antigens and proteins naturally present on platelet membranes. This is unlike other efforts, which synthesize platelet mimics that replicate one or two surface proteins of the platelet membrane.
"Our technique takes advantage of the unique natural properties of human platelet membranes, which have a natural preference to bind to certain tissues and organisms in the body," said Zhang. This targeting ability, which red blood cell membranes do not have, makes platelet membranes extremely useful for targeted drug delivery, researchers said.
Platelet copycats at work
In one part of this study, researchers packed platelet-mimicking nanoparticles with docetaxel, a drug used to prevent scar tissue formation in the lining of damaged blood vessels, and administered them to rats afflicted with injured arteries. Researchers observed that the docetaxel-containing nanoparticles selectively collected onto the damaged sites of arteries and healed them.
When packed with a small dose of antibiotics, platelet-mimicking nanoparticles can also greatly minimize bacterial infections that have entered the bloodstream and spread to various organs in the body. Researchers injected nanoparticles containing just one-sixth the clinical dose of the antibiotic vancomycin into one of group of mice systemically infected with MRSA bacteria. The organs of these mice ended up with bacterial counts up to one thousand times lower than mice treated with the clinical dose of vancomycin alone.
"Our platelet-mimicking nanoparticles can increase the therapeutic efficacy of antibiotics because they can focus treatment on the bacteria locally without spreading drugs to healthy tissues and organs throughout the rest of the body," said Zhang. "We hope to develop platelet-mimicking nanoparticles into new treatments for systemic bacterial infections and cardiovascular disease."
###
This work is supported by the National Institutes of Health and partially by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense. The collaborative effort also includes Kang Zhang, a professor of ophthalmology and chief of Ophthalmic Genetics at UC San Diego and a corresponding author on this study.
Full paper: "Nanoparticle biointerfacing by platelet membrane cloaking" by Che-Ming J. Hu, Ronnie H. Fang, Kuei-Chun Wang, Brian T. Luk, Soracha Thamphiwatana, Diana Dehaini, Phu Nguyen, Pavimol Angsantikul, Cindy H. Wen, Ashlev V. Kroll, Cody Carpenter, Manikantan Ramesh, Vivian Qu, Sherrina H. Patel, Jie Zhu, William Shi, Florence M. Hofman, Thomas C. Chen, Weiwei Gao, Kang Zhang, Shu Chien, and Liangfang Zhang. This paper is published Sept. 16, 2015 online in Nature.
####
For more information, please click here
Contacts:
Liezel Labios
858-246-1124
Copyright © University of California - San Diego
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








