Home > Press > Nanoparticles treat muscular dystrophy in mice
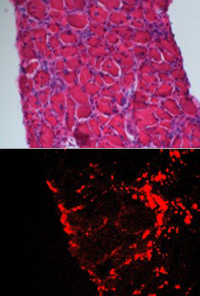 |
| FASEB Journal Shown is a diaphragm muscle of a mouse with muscular dystrophy (top). Pictured below are fluorescent nanoparticles (red) lodged in the diaphragm. |
Abstract:
Researchers at Washington University School of Medicine in St. Louis have demonstrated a new approach to treating muscular dystrophy. Mice with a form of this muscle-weakening disease showed improved strength and heart function when treated with nanoparticles loaded with rapamycin, an immunosuppressive drug recently found to improve recycling of cellular waste.
Nanoparticles treat muscular dystrophy in mice
St. Louis, MO | Posted on February 11th, 2014The study appears online in The FASEB Journal.
The investigators, including first author Kristin P. Bibee, MD, PhD, looked at a mouse model of Duchenne muscular dystrophy, the most severe inherited form of the disease. Duchenne exclusively affects boys who have to rely on wheelchairs by age 12 and die from heart or respiratory failure in their 20s.
The faulty gene that causes the disease prevents the body from producing dystrophin, a protein crucial for maintaining muscle cell integrity and function. The new study demonstrated that mice with muscular dystrophy, in addition to missing dystrophin, also can't recycle cellular waste, a process known as autophagy, or self-eating.
"Autophagy plays a major role in disposing of cellular debris," said senior author Samuel A. Wickline, MD, the James R. Hornsby Family Professor of Medicine. "If it doesn't happen, you might say the cell chokes on its own refuse. In muscular dystrophy, defective autophagy is not necessarily a primary source of muscle weakness, but it clearly becomes a problem over time. If you solve that, you can help the situation by maintaining more normal cellular function."
Though it's not clear how the missing dystrophin protein might be responsible for the muscle cells' poor housekeeping, the study showed that boosting autophagy improved skeletal muscle strength and heart function in these mice.
"Some investigators are looking for ways to replace dystrophin," said co-author Conrad C. Weihl, MD, PhD, associate professor of neurology. "But here we are focusing on the defect in autophagy. What is exciting about our approach is that there are existing drugs that activate autophagy. And by repackaging the drug on nanoparticles, we can target it specifically to muscles and correct the defect in the cells' ability to recycle waste."
When treated with rapamycin nanoparticles, the mice showed a 30 percent increase in grip strength and a significant improvement in cardiac function, based on an increase in the volume of blood the heart pumped.
"An important aspect of our study is that we are treating both skeletal muscle and heart muscle with the same drug," Wickline said. "The heart is a difficult organ to treat in muscular dystrophy. But even in older animals, this regimen works well to recover heart function, and it is effective over a short period of time and after only a few doses."
"Death from Duchenne in many people is due to heart dysfunction," said Weihl, who also treats patients with neuromuscular disorders at Barnes-Jewish Hospital. "So even improving cardiac function by 10 percent in late-stage disease could be very important."
The nanoparticle used in the study consists of an inert core made of perfluorocarbon, originally designed as a blood substitute. The particles are about 200 nanometers in diameter—500 times smaller than the thickness of a human hair. The surface of the nanoparticle is coated with rapamycin, which suppresses the immune system. The drug typically is used to help prevent organ rejection in transplant patients. It is known for its anti-inflammatory properties and, more recently, for its role in activating autophagy.
When injected into the bloodstream, according to Wickline, the nanoparticles accumulate in areas of inflammation, where damaged tissues have leaky blood vessels and are undergoing cell death and repair.
"The nanoparticles tend to penetrate and be retained in areas of inflammation," Wickline said. "Then they release the rapamycin over a period of time, so the drug itself can permeate the muscle tissue."
Compared with oral delivery, the nanoparticle approach also allowed the researchers to give the mice smaller doses of the drug.
"We showed that oral doses of rapamycin, even at 10 times the dose we used in the nanoparticles, were ineffective," Weihl said. "This is important because rapamycin suppresses the immune system, and directly targeting it to muscle in smaller doses would reduce unwanted side effects."
Current treatment for Duchenne involves corticosteroids such as prednisone, which has been shown to extend the time patients are able to walk. But steroids also cause weight gain, brittle bones, high blood pressure and other long-term side effects.
Although it's not clear why steroid treatment helps maintain skeletal muscle strength, Weihl said the study suggests prednisone also may promote autophagy, raising the possibility of combination therapy, in which both steroid treatment and rapamycin nanoparticles could be given simultaneously, each at lower doses.
This work was supported by the National Institutes of Health (NIH) grants R01 AR056223, HL073646, HL112518, HL113392, NS073457ß, K02 AG042095, the Muscular Dystrophy Association, and the American Heart Association.
Bibee KP, Cheng YJ, Ching JK, Marsh JN, Li AJ, Keeling RM, Connolly AM, Golumbek PT, Myerson JW, Hu G, Chen J, Shannon WD, Lanza GM, Weihl CC, Wickline SA. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. The FASEB Journal. Online Feb. 5, 2013.
####
About Washington University in St. Louis
Washington University School of Medicine’s 2,100 employed and volunteer faculty physicians also are the medical staff of Barnes-Jewish and St. Louis Children’s hospitals. The School of Medicine is one of the leading medical research, teaching and patient-care institutions in the nation, currently ranked sixth in the nation by U.S. News & World Report. Through its affiliations with Barnes-Jewish and St. Louis Children’s hospitals, the School of Medicine is linked to BJC HealthCare.
For more information, please click here
Contacts:
Julia Evangelou Strait
Senior Medical Sciences Writer
(314) 286-0141
Copyright © Washington University in St. Louis
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








