Home > Press > New technique targets specific areas of cancer cells with different drugs
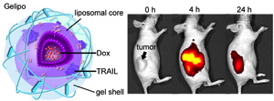 |
| Image shows the structure of the nanoparticle (left), and how the nanoparticles home in on a tumor and shrink it |
Abstract:
"Gel-Liposome-Mediated Co-Delivery of Anticancer Membrane-Associated Proteins and Small-Molecule Drugs for Enhanced Therapeutic Efficacy"
Authors: Tianyue Jiang, Ran Mo, and Zhen Gu, North Carolina State University and University of North Carolina at Chapel Hill; Adriano Bellotti, North Carolina State University; Jianping Zhou, China Pharmaceutical University.
Published: online Jan. 2, 2014, Advanced Functional Materials
Abstract: A programmed drug-delivery system that can transport different anticancer therapeutics to their distinct targets holds vast promise for cancer treatment. Herein, a core-shell-based "nanodepot" consisting of a liposomal core and a crosslinked-gel shell (designated Gelipo) is developed for the sequential and site-specific delivery (SSSD) of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and doxorubicin (Dox). As a small-molecule drug intercalating the nuclear DNA, Dox is loaded in the aqueous core of the liposome, while TRAIL, acting on the death receptor (DR) on the plasma membrane, is encapsulated in the outer shell made of crosslinked hyaluronic acid (HA). The degradation of the HA shell by HAase that is concentrated in the tumor environment results in the rapid extracellular release of TRAIL and subsequent internalization of the liposomes. The parallel activity of TRAIL and Dox show synergistic anticancer efficacy. The half-maximal inhibitory concentration (IC50) of TRAIL and Dox co-loaded Gelipo (TRAIL/Dox-Gelipo) toward human breast cancer (MDA-MB-231) cells is 83 ng mL-1 (Dox concentration), which presents a 5.9-fold increase in the cytotoxicity compared to 569 ng mL-1 of Dox-loaded Gelipo (Dox-Gelipo). Moreover, with the programmed choreography, Gelipo significantly improves the inhibition of the tumor growth in the MDA-MB-231 xenograft tumor animal model.
New technique targets specific areas of cancer cells with different drugs
Raleigh, NC | Posted on January 6th, 2014Researchers have developed a technique for creating nanoparticles that carry two different cancer-killing drugs into the body and deliver those drugs to separate parts of the cancer cell where they will be most effective. The technique was developed by researchers at North Carolina State University and the University of North Carolina at Chapel Hill.
"In testing on laboratory mice, our technique resulted in significant improvement in breast cancer tumor reduction as compared to conventional treatment techniques," says Dr. Zhen Gu, senior author of a paper on the research and an assistant professor in the joint biomedical engineering program at NC State and UNC-Chapel Hill.
"Cancer cells can develop resistance to chemotherapy drugs, but are less likely to develop resistance when multiple drugs are delivered simultaneously," Gu says. "However, different drugs target different parts of the cancer cell. For example, the protein drug TRAIL is most effective against the cell membrane, while doxorubicin (Dox) is most effective when delivered to the nucleus. We've come up with a sequential and site-specific delivery technique that first delivers TRAIL to cancer cell membranes and then penetrates the membrane to deliver Dox to the nucleus."
Gu's research team developed nanoparticles with an outer shell made of hyaluronic acid (HA) woven together with TRAIL. The HA interacts with receptors on cancer cell membranes, which "grab" the nanoparticle. Enzymes in the cancer cell environment break down the HA, releasing TRAIL onto the cell membrane and ultimately triggering cell death.
When the HA shell breaks down, it also reveals the core of the nanoparticle, which is made of Dox that is embedded with peptides that allow the core to penetrate into the cancer cell. The cancer cell encases the core in a protective bubble called an endosome, but the peptides on the core cause the endosome to begin breaking apart. This spills the Dox into the cell where it can penetrate the nucleus and trigger cell death.
"We designed this drug delivery vehicle using a ‘programmed' strategy," says Tianyue Jiang, a lead author in Dr. Gu's lab. "Different drugs can be released at the right time in their right places," adds Dr. Ran Mo, a postdoctoral researcher in Gu's lab and the other lead author.
"This research is our first proof of concept, and we will continue to optimize the technique to make it even more efficient," Gu says. "The early results are very promising, and we think this could be scaled up for large-scale manufacturing."
-shipman-
####
For more information, please click here
Contacts:
Matt Shipman
919-515-6386
Dr. Zhen Gu
919.515.7944
Copyright © North Carolina State University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Industrial
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Quantum interference in molecule-surface collisions February 28th, 2025
Quantum interference in molecule-surface collisions February 28th, 2025
![]() Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
Boron nitride nanotube fibers get real: Rice lab creates first heat-tolerant, stable fibers from wet-spinning process June 24th, 2022
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








