Home > Press > Cartilage damaged from exercise may aid in early osteoarthritis detection
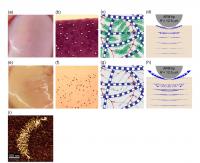 |
| These are images of (a) normal human joint cartilage, and (e) cartilage with very early matrix GAG loss. (b) Histologic image of a normal cartilage stained with Toluidine Blue to visualize content of GAGs. (f) Histologic image of a GAG-depleted cartilage (c) Schematic of extracellular matrix of normal cartilage composed mainly of collagen fibers and aggrecan (g) Schematic of matrix of GAG-depleted cartilage (d,h) Schematic of AFM-based dynamic compression of normal (d) and GAG-depleted (h) cartilage, which results in intra-tissue fluid flow velocity depicted by the blue arrows (from Finite element model computer simulations): loss of GAG (h) enables more fluid to flow out of the cartilage at high loading rates. (i) Human aggrecan imaged by Atomic Force Microscopy (AFM)
Credit: Biophysical Journal, Nia et al. |
Abstract:
Osteoarthritis is the most common joint disorder, affecting about one-third of older adults, and currently there is no cure. A study published by Cell Press April 2nd in the Biophysical Journal reveals how the nanoscale biomechanical properties of cartilage at joints change at the earliest stages of osteoarthritis, making the tissue more prone to damage during fast physical activities. The findings could improve early detection of the disease as well as tissue engineering strategies to repair damaged cartilage in patients.
Cartilage damaged from exercise may aid in early osteoarthritis detection
Cambridge, MA | Posted on April 2nd, 2013"Our techniques enable detection of the earliest loss of mechanical function associated with daily activities involving high loading rates, such as running and jumping," says senior study author Alan Grodzinsky of the Massachusetts Institute of Technology. "The findings can also be used to evaluate replacement tissue to ensure that it can survive these daily activities."
Osteoarthritis is a painful condition marked by the deterioration of cartilage—firm, rubbery tissue that cushions bones and prevents them from rubbing together. At the earliest stages of the disease, cartilage loses molecules called glycosaminoglycans (GAGs), which reduces the ability of the tissue to resist impact caused by physical activity. But until now, little was known about how GAG loss affects the functioning of cartilage across a wide spectrum of activities, from walking to running and jumping.
To address this question, Grodzinsky and his team developed a new system to measure the biomechanical properties of cartilage in response to cyclic compression forces that simulated a range of physical activities, each occurring at a different timescale. GAG-depleted cartilage was less capable of increasing its stiffness to deal with forces associated with high-rate activities such as running, when compared with normal tissue. Moreover, GAG loss resulted in a dramatic increase in the ability of fluids to flow out of cartilage, which is expected to diminish protection against impact caused by fast activities.
Together, the findings show how GAG depletion at the earliest disease stages could affect the nanoscale properties of cartilage, reducing the ability of this tissue to withstand high-rate activities. "We discovered that GAG-depleted tissue is most vulnerable to high rates of loading and not just the magnitude of the load," Grodzinsky says. "This finding suggests that people with early degradation of cartilage, even before such changes would be felt as pain, should be careful of dynamic activities such as running or jumping."
Biophysical Journal, Nia et al.: "High-Bandwidth AFM-Based Rheology Reveals that Cartilage is Most Sensitive to High Loading Rates at Early Stages of Impairment."
####
For more information, please click here
Contacts:
Mary Beth O'Leary
617-397-2802
Copyright © Cell Press
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








