Home > Press > A new look at proteins in living cells
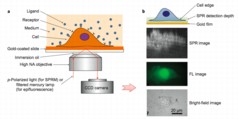 |
| Figure 1a: Schematic illustration of the experimental set-up for surface plasmon resonance microscopy. A polarized laser beam is directed onto a gold-coated glass coverslip through an oil-immersion objective to create SPR on the gold surface, which is imaged with a CCD camera. 1b: From the bottom up, examples of bright-field, fluorescence (FL) and SPR images, respectively. |
Abstract:
Proteins adorning the surfaces of human cells perform an array of essential functions, including cell signaling, communication and the transport of vital substances into and out of cells. They are critical targets for drug delivery and many proteins are now being identified as disease biomarkers—early warning beacons announcing the pre-symptomatic presence of cancers and other diseases.
A new look at proteins in living cells
Tempe, AZ | Posted on August 28th, 2012While study of the binding properties of membrane proteins is essential, detailed analysis of these complex entities is tricky. Now, Nongjian (NJ) Tao, Professor of Electrical Engineering, and director of the Center for Bioelectronics and Biosensors at Arizona State University's Biodesign Institute has devised a new technique for examining the binding kinetics of membrane proteins.
"This is a very important but very difficult problem to solve," Tao notes. "We demonstrate a new method of approaching the issue, which provides a quantitative analysis of protein interactions on the surface of a cell."
The technique—known as SPR microscopy—holds the potential to simplify the study of membrane proteins, thereby streamlining the development of new drugs, aiding the identification of diagnostic biomarkers and improving the understanding of cell-pathogen interactions.
The group's results appear in this week's advanced online issue of the journal Nature Chemistry.
Typically, proteins attached to or embedded in the cell membrane's lipid bilayer are either tagged with fluorescent markers or extracted from their locations, purified and immobilized on a glass surface in protein microarrays. These efforts may not accurately reflect native configuration and function.
Membrane proteins are complex structures whose subtle performance is often related to alterations in conformation and the particular binding kinetics at work. Existing techniques using florescent markers have been applied to pinpoint binding events, but these only permit the visualization of the protein before and after binding, omitting the dynamic processes evolving over time. Further, the use of fluorescent labels to tag protein molecules can interfere with the processes researchers hope to observe.
Alternately, proteins are extracted, purified and affixed to microarray slides—a labor-intensive process that removes proteins from their native environment, potentially affecting the shapes they naturally assume in situ and/or altering protein function.
In the current study, a label-free imaging technique is applied in situ to membrane proteins, which are visualized using a property known as surface plasmon resonance. This effect occurs when polarized light strikes the surface of a glass slide coated with a thin metallic film of gold. Under proper conditions of wavelength, polarization and incident angle, free electrons in the metal film absorb incident photons, converting them into plasmon waves, which propagate much like waves in water.
When nanoscale phenomena, including membrane proteins, interact and disrupt plasmon waves, they cause a measurable change in light reflectivity, which the new microscopy method converts into an image. (Figure 1a illustrates the basic setup of this technique.)
Surface plasmon resonance had already been applied to extracted proteins to study binding kinetics, though Tao explains that many steps are required and proteins may lose their proper conformational characteristics. This is particularly true for proteins normally embedded in a cell membrane's lipid matrix.
Another important consideration for the study of membrane proteins is the fact that that they arrange themselves heterogeneously across membrane surfaces and modify their distribution during various cellular activities. This behavior is particularly important during a process known as chemotaxis, when cells direct their movements under the influence of chemicals in the surrounding environment. For this reason, a tool allowing for both spatial and temporal study of membrane protein distribution in real time is highly desirable.
Tao's method uses surface plasmon resonance to provide high-resolution spatial and temporal information, and also allows for simultaneous optical and fluorescence observation of the sample, combining the advantages of both label-based and label-free methods.
High spatial resolution proved particularly useful for observing the ways polarized membrane proteins (bearing hydrophobic and hydrophilic regions) rearrange themselves, assisting cell migration directed by surrounding chemicals. The phenomenon also plays an important role during immune recognition. Using SPR microscopy, the spatial distribution of membrane proteins in single cells during chemotaxis could be mapped in detail for the first time, using a chemoattractant to induce cell migration.
Cells for study are cultured directly on a gold-coated slide, which can be subjected to simultaneous bright-field, florescent and SPR imaging. A liquid containing binding ligands is then applied over cells and the binding events with cell surface proteins monitored with SPR.
The technique permits millisecond resolution of temporal events and sub-micron scale analysis of spatial distribution. (See Figure 1b). In the current study, the method examined the binding of membrane glycoproteins with lectin ligands, the spatial distribution of membrane receptor molecules and membrane protein polarization and redistribution events.
The versatility of the new method, allowing for simultaneous imaging in optical, fluorescent and SPR modes, promises to significantly expand the study of membrane proteins in their native state, improving the understanding of protein binding kinetics and speeding the development of drugs targeting membrane proteins.
Tao stresses that such techniques—by more closely approximating in vivo conditions— provide a valuable window into biological processes relevant to health and disease: "Cells are different from tissues which are different from human beings, but at least now we can move from a system on the surface of a glass slide to an actual cell surface."
In addition to his appointment as the Director of the Center for Bioelectronics and Biosensors at the Biodesign Institute, Tao is Ira A. Fulton School of Engineering, School of Electrical, Computer and Energy Engineering.
####
For more information, please click here
Contacts:
Joseph Caspermeyer
Copyright © Arizona State University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








