Home > Press > Force of nature: Defining the mechanical mechanisms in living cells: Exploring mechanical forces at the nanoscale, researchers show cadherin-catenin-actin structure exerts force inside and between cells in living tissues
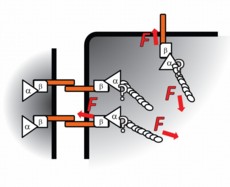 |
| Dunn's proposed model: Cadherin (orange bars) transcends the cell membrane (thick black lines), binding cells to one another. Inside the cell, cadherin is under tension from actomyosin (ellipsoid spirals) connected by alpha-catenin (α) and beta-catenin (β). Illustration courtesy of Nicolas Borghi, Stanford University School of Engineering. |
Abstract:
If you place certain types of living cells on a microscope slide, the cells will inch across the glass, find their neighbors, and assemble themselves into a simple, if primitive tissue. A new study at Stanford University may help explain this phenomenon, and then some, about the mechanical structure and behavior of complex living organisms.
Force of nature: Defining the mechanical mechanisms in living cells: Exploring mechanical forces at the nanoscale, researchers show cadherin-catenin-actin structure exerts force inside and between cells in living tissues
Stanford, CA | Posted on July 16th, 2012In the paper published in the Proceedings of the National Academy of Sciences, chemical engineer Alexander Dunn, PhD, and a multidisciplinary team of researchers in biology, physiology, and chemical and mechanical engineering, were able to measure—and to literally see—the mechanical forces at play between and within the living cells.
There are scads of data explaining chemical signaling between cells. "And yet, one of the great roadblocks to a complete knowledge of how cells work together to form tissues, organs and, ultimately, us, is an understanding of the mechanical forces at play between and within cells," said Dunn.
Using a new force-sensing technique, Dunn and team have been able to see mechanical forces at work inside living cells to understand how cells connect to one another and how individual cells control their own shape and movement within larger tissues.
Pulling back the veil on the exact nature of this mechanism could have bearing on biological understanding ranging from how tissues and tumors form and grow, to the creation of entire complex living organisms.
Seeing the force
"Cells are really just machines. Small, incredibly complex biological machines, but machines nonetheless," said Dunn. "They rely on thousands of moving parts that give the cell shape and control of its destiny."
The mechanical parts are proteins whose exact functions often remain a mystery, but Dunn and team have helped explain the behaviors of a few.
At its most basic level, a cell is like a balloon filled with saltwater, Dunn explained. The exterior of the cell, the balloon part, is known as the membrane. Protruding through the membrane, with portions both inside and outside the cell, are certain proteins called cadherins.
Outside of the cell, cadherins bind one cell to its neighbors like Velcro. The 'herin' portion of the name, in fact, shares a Latin root with "adhere."
On the inside of the cell, cadherin is connected to long fibers of actin and myosin that stretch from membrane to nucleus to membrane again. Actin and myosin work together as the muscle of the cell, providing tension that gives the cell shape and the ability to control its own movement. Without this force, the balloon of the cell would be a shapeless, immobile blob.
Puppeteer's string
"If you watch a cell moving across a glass slide, you can see it attach itself on one side of the cell and detach on the other, which causes a contraction that allows the cell to, bit by bit, pull itself from place to place," said Dunn. "It's clearly moving itself."
While it was understood that cadherin and actin are connected to one another by other proteins known as catenins, what was not known was how, when, and where the cells might be using their muscles (actin and myosin) to tug on the Velcro (cadherin) that holds them to other cells.
This is an important problem in the development of organisms, since a cell must somehow control its shape and its attachments to other cells as it grows, divides, and migrates from one place to another within the tissue. Dunn and his colleagues have shown that the actin-catenin-cadherin structure transmits force within the cell and, further, that cadherin can convey mechanical forces from one cell to the next.
It is a form of mechanical communication, like the strings of a puppeteer. Dunn and others in the field believe that these mechanical forces may be important in conveying to a cell how to position itself within a tissue, when to reproduce and when to stop as the tissue reaches its proper size and shape.
"That is the theory, but an important piece was missing," said Dunn. "Our research shows that forces at cell-cell contacts can in fact be communicated from one cell to its neighbors. The theorized mechanical signaling mechanism is feasible."
Story within a story
How Dunn and his colleagues got to this point is a story in itself. It reads like the recipe for a witch's potion—cultured canine kidney cells, DNA from jellyfish and spider silk, and microscopic glass needles.
To measure the force between cells, a team combining the skill of several Stanford laboratories—headed by Professor Dunn in chemical engineering, professors William Weis and W. James Nelson in the Department of Molecular and Cellular Physiology and associate professor of mechanical engineering Beth Pruitt—used a tiny and ingenious molecular force sensor developed by Martin Schwartz and colleagues at the University of Virginia. The sensor combines fluorescent proteins from jellyfish with a springy protein from spider silk.
The genes for the sensor are incorporated into the cell's DNA. Under illumination, the cells glow in varying colors depending on how much stretch the sensor is under. In this study, the force sensor is inserted into the cadherin molecules—when the Velcro stretches, so does the sensor.
The team then took things a step further. By turning the activity of myosin, actin and catenin on and off, they were able to determine that these proteins are in fact linked together and are at the heart of inter- and intra-cellular mechanical force transmission.
Lastly, using glass microneedles, the team tugged at connected pairs of cells, pulling at one cell to show that force gets communicated to the other through the cadherin interface.
"At this point we now know that a cell exerts exquisite control over the balance of its internal forces and can detect force exerted from outside by its neighbors, but we still know next to nothing about how," said Dunn. "We are extremely curious to find out more."
Post-doctoral scholar Nicolas Borghi, laboratory technician Maria Sorokina and staff scientist Olga Shcherbakova were co-first authors on the paper.
This research was made possible by funding from a Stanford Bio-X IIP award, the National Science Foundation, the National Institutes of Health and a Burroughs-Wellcome Career Award at the Scientific Interface.
This article was written by Andrew Myers, associate director of communications for the Stanford University School of Engineering.
####
For more information, please click here
Contacts:
Andrew Myers
650-736-2241
Copyright © Stanford School of Engineering
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Grants/Sponsored Research/Awards/Scholarships/Gifts/Contests/Honors/Records
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
Researchers tackle the memory bottleneck stalling quantum computing October 3rd, 2025
![]() New discovery aims to improve the design of microelectronic devices September 13th, 2024
New discovery aims to improve the design of microelectronic devices September 13th, 2024
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








