Home > Press > Atomic-scale Structures of Ribosome Could Help Improve Antibiotics: Berkeley Lab scientists reveal how protein-making machine bends without breaking
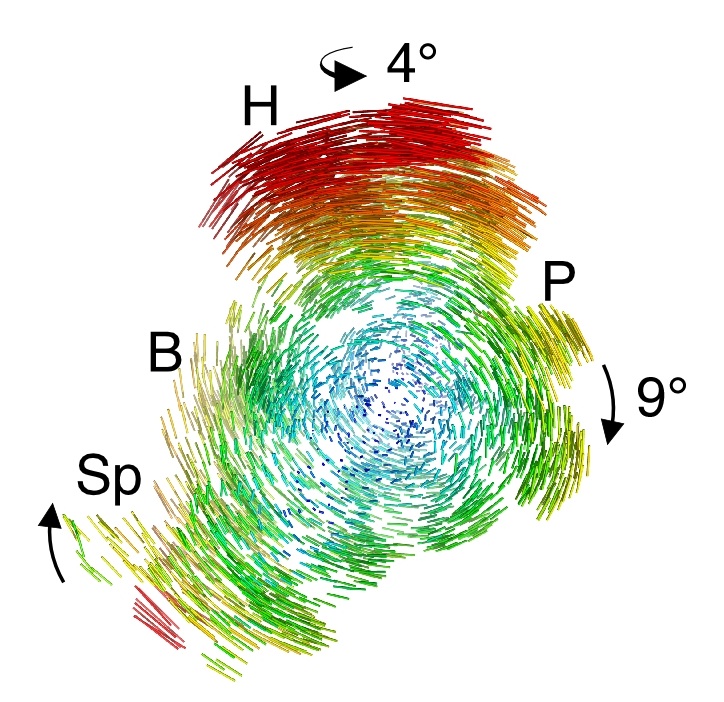 |
| This “action shot” reveals the motion of the small ribosomal subunit, depicted by difference vectors, during ratcheting. |
Abstract:
It sounds like hype from a late-night infomercial: It can twist and bend without breaking! And wait, there's more: It could someday help you fend off disease!
But in this case it's true, thanks to scientists from several institutions including the U.S. Department of Energy's Lawrence Berkeley National Laboratory. They derived atomic-scale resolution structures of the cell's protein-making machine, the ribosome, at key stages of its job.
Atomic-scale Structures of Ribosome Could Help Improve Antibiotics: Berkeley Lab scientists reveal how protein-making machine bends without breaking
Berkeley, CA | Posted on May 20th, 2011The structures, developed primarily at Berkeley Lab's Advanced Light Source, reveal that the ribosome's ability to rotate an incredible amount without falling apart is due to the never-before-seen springiness of molecular widgets that hold it together.
The structures also provide an atom-by-atom map of the ribosome when it's fully rotated during the final phase of protein synthesis. Many antibiotics target the ribosomes of disease-causing microbes at precisely this stage. The high-resolution structures could allow scientists to develop antibiotics that better target this cellular Achilles' heel, perhaps leading to drugs that are less susceptible to resistance.
"Parts of the ribosome are much more flexible than we previously thought. In addition, now that we have a fully rotated ribosomal structure, scientists may be able to develop new antibiotics that are not as sensitive to ribosomal mutations. This could help mitigate the huge problem of multidrug resistance," says Jamie Cate, a staff scientist in Berkeley Lab's Physical Biosciences Division and an associate professor of biochemistry, molecular biology, and chemistry at the University of California at Berkeley.
Cate conducted the research with a team that includes scientists from Cornell University and Duke University. Their research is published in the May 20 issue of the journal Science.
The ribosome works like a protein assembly line. Its smaller subunit moves along messenger RNA, which contributes genetic information from the cell's DNA. The smaller subunit also binds to transfer RNA, which connect the genetic code on one end with amino acids on the other. The amino acids are stitched together into proteins by the larger subunit, which also binds to the transfer RNA. In this way, the two ribosomal subunits come together to create proteins that conduct the heavy lifting in the cells of all living things, from bacteria to trees to humans.
Scientists have used biochemistry and low-resolution electron microscopy to map much of the ribosome's structural changes throughout its protein-making cycle. But key steps remained unclear, such as a ratchet-like motion of the small ribosomal subunit relative to the large subunit as it moves in one direction along the messenger RNA to make a protein. These parts rotate relative to another, but scientists didn't know how this large-scale twisting motion worked in molecular detail — or why it didn't simply wrench the entire ribosome apart.
To find out, the scientists turned to the Advanced Light Source, a synchrotron located at Berkeley Lab that generates intense x-rays to probe the fundamental properties of molecules. Using beamline 8.3.1 and the SIBYLS beamlines, they determined the structure of Escherichia coli ribosomes in two key states for the first time at an atomic-scale resolution. In the first state, transfer RNA is bound to the two subunits in a configuration that occurs after the ribosome has made and released a protein. In the second state, the ribosome's subunits are fully rotated, which occurs when the subunits are recycled and ready to make another protein. The scientists used x-ray crystallography to piece together these structures at a resolution of approximately 3.2 Ångstroms (one Ångstrom is a ten-billionth of a meter, about the radius of the smallest atoms).
The resulting structures, which are two to three times higher resolution than previous images of the ribosome at these states, capture the inner-workings of the ribosome like never before. They reveal that the ribosome machine contains molecular-scale compression springs and torsion springs made of RNA. These molecular springs keep the ribosome's subunits tethered together even as they rotate with respect to each other.
"This is first time we've seen the ribosome at the endpoint of this motion at this resolution," says Cate. "And the question is, when you have these big motions, why doesn't the ribosome fall apart. We found that the ribosome has RNA springs that adjust their shape and allow it to stay together during these large-scale motions."
The structures also provide a new way to compete in the arms race between antibiotics and the microbes they're designed to knock out.
"The ribosome is one of the major targets of antibiotics, and we've identified elements of its rotation that can be targeted by new or modified antibiotics," says Cate. "This kind of precision could be especially powerful in the age of personalized medicine. Scientists could figure out at a genetic level why someone isn't responding to an antibiotic, and then possibly switch to a more effective antibiotic that better targets the microbe that's causing their disease."
The research was supported by the National Institutes of Health's Institute of General Medical Sciences. The Advanced Light Source and beamline 8.3.1 and SIBYLS beamline are supported by the Department of Energy's Office of Science. This research was also conducted at the DOE Office of Science-supported Advanced Photon Source located at Argonne National Laboratory.
####
About Berkeley Lab
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 12 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
For more information, please click here
Contacts:
Dan Krotz
510-486-4019
Copyright © Berkeley Lab
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() More about the Advanced Light Source:
More about the Advanced Light Source:
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Videos/Movies
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Research partnerships
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








