Home > Press > UCLA nanotech research mimics enzymes in directing chemical reactions: New method for studying molecule reactions a breakthrough in organic chemistry
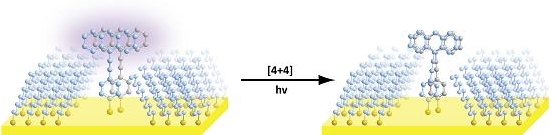 |
| Two molecules are placed in proximity in "cutouts" in self-assembled monolayers. When excited with ultraviolet light, they are constrained to react along a pathway different than they would if they could reorient in solution. |
Abstract:
Good chemists are passive-aggressive - they manipulate molecules without actually touching them.
In a feat of manipulating substances at the nanoscale, UCLA researchers and colleagues demonstrated a method for isolating two molecules together on a substrate and controlling how those two molecules react when excited with ultraviolet light, making detailed observations both before and after the reaction.
UCLA nanotech research mimics enzymes in directing chemical reactions: New method for studying molecule reactions a breakthrough in organic chemistry
Los Angeles, CA | Posted on March 11th, 2011Their research is published today in the journal Science.
"This is one step in measuring and understanding the interactions between light and molecules, which we hope will eventually lead to more efficient conversion of sunlight to electrical and other usable forms of energy," said lead study author Paul S. Weiss, a distinguished professor of chemistry and biochemistry who holds UCLA's Fred Kavli Chair in Nanosystems Sciences. "Here, we used the energy from the light to induce a chemical reaction in a way that would not happen for molecules free to move in solution; they were held in place by their attachment to a surface and by the unreactive matrix of molecules around them."
Weiss is also director of UCLA's California NanoSystems Institute (CNSI) and a professor of materials science and engineering at the UCLA Henry Samueli School of Engineering and Applied Science.
Controlling exactly how molecules combine in order to study the resulting reactions is called regioselectivity. It is important because there are a variety of ways that molecules can combine, with varying chemical products. One way to direct a reaction is to isolate molecules and to hold them together to get regioselective reactions; this is the strategy used by enzymes in many biochemical reactions.
"The specialized scanning tunneling microscope used for these studies can also measure the absorption of light and charge separation in molecules designed for solar cells," Weiss said. "This gives us a new way to optimize these molecules, in collaboration with synthetic chemists. This is what first brought us together with our collaborators at the University of Washington, led by Prof. Alex Jen."
Alex K-Y. Jen holds the Boeing-Johnson Chair at the University of Washington, where he is a professor of materials science and engineering and of chemistry. The theoretical aspects of the study were led by Kendall Houk, a UCLA professor of chemistry and biochemistry who holds the Saul Winstein Chair in Organic Chemistry. Houk is a CNSI researcher.
The study's first author, Moonhee Kim, a graduate student in Weiss' lab, managed to isolate and control the reactions of pairs of molecules by creating nanostructures tailored to allow only two molecules fit in place. The molecules used in the study are photosensitive and are used in organic solar cells; similar techniques could be used to study a wide variety of molecules. Manipulating the way molecules in organic solar cells come together may also ultimately lead to greater efficiency.
To isolate the two molecules and align them in the desired - but unnatural - way, Kim utilized a concept similar to that of toddler's toys that feature cutouts in which only certain shapes will fit.
She created a defect, or cutout, in a self-assembled monolayer, or SAM, a single layer of molecules on a flat surface - in this case, gold. The defect in the SAM was sized so that only two organic reactant molecules would fit and would only attach with the desired alignment. As a guide to attach the molecules to the SAM in the correct orientation, sulfur was attached to the bottoms of the molecules, as sulfur binds readily to gold.
"The standard procedure for this type of chemistry is to combine a bunch of molecules in solution and let them react together, but through random combinations, only 3 percent of molecules might react in this way," UCLA's Houk said. "Our method is much more targeted. Instead of doing one measurement on thousands of molecules, we are doing a range of measurements on just two molecules."
After the molecules were isolated and trapped on the substrate, they still needed to be excited with light to react. In this case, the energy was supplied by ultraviolet light, which triggered the reaction. The researchers were able to verify the proper alignment and the reaction of the molecules using the special microscope developed by Kim and Weiss.
The work was funded by the U.S. Department of Energy, the National Science Foundation, the Air Force Office of Scientific Research and the Kavli Foundation.
####
For more information, please click here
Copyright © UCLA
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Chip Technology
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() Beyond silicon: Electronics at the scale of a single molecule January 30th, 2026
Beyond silicon: Electronics at the scale of a single molecule January 30th, 2026
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Self Assembly
![]() Diamond glitter: A play of colors with artificial DNA crystals May 17th, 2024
Diamond glitter: A play of colors with artificial DNA crystals May 17th, 2024
![]() Liquid crystal templated chiral nanomaterials October 14th, 2022
Liquid crystal templated chiral nanomaterials October 14th, 2022
![]() Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
Nanoclusters self-organize into centimeter-scale hierarchical assemblies April 22nd, 2022
![]() Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Atom by atom: building precise smaller nanoparticles with templates March 4th, 2022
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








