Home > Press > Researchers First to "See" Reactive Oxygen Species in Vital Enzyme: Mechanistic details revealed through unique light source technique
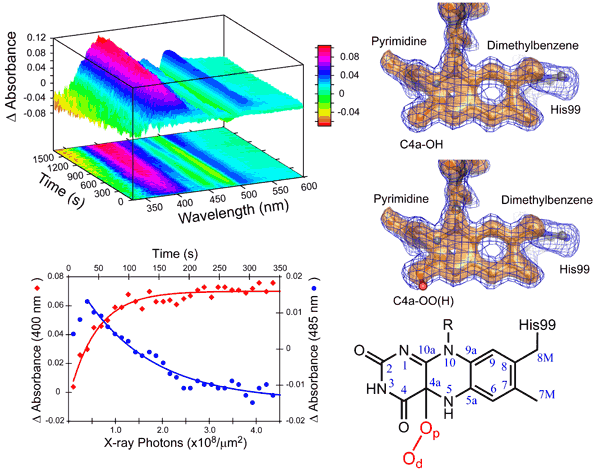 |
| Two complementary types of data collected from the same crystal provide compelling evidence for the trapped reactive oxygen intermediate in choline oxidase. On the left are the spectroscopic changes observed in a single crystal of choline oxidase upon x-ray exposure at low temperature. It shows that the sample rapidly changes upon irradiation to x-rays. On the right are the unbiased 1.8 angstrom resolution electron density maps and the resulting interpretation of the atomic structures for the two possible reactive oxygen species. The electronic and atomic structures correlate almost perfectly within the crystal. In the absence of either part of the complementary experimental data, the interpretation of the results would have remained much more speculative. |
Abstract:
Using two simultaneous light-based probing techniques at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, a team of researchers has illuminated important details about a class of enzymes involved in everything from photosynthesis to the regulation of biological clocks.
Researchers First to "See" Reactive Oxygen Species in Vital Enzyme: Mechanistic details revealed through unique light source technique
Upton, NY | Posted on January 10th, 2009The interdisciplinary team has a broad interest in flavoproteins, which were first discovered in the 1930s and derive from riboflavin, or vitamin B2. These proteins are now known to catalyze a wide range of biochemical reactions, including those that use molecular oxygen (O2) to help convert food into energy in animals, plants, fungi, and in some types of bacteria - a process known as oxygen activation.
Although scientists have determined more than 1,200 crystal structures of flavoproteins, they've been blind to exactly what oxygen activation looks like within these enzymes. Specifically, researchers have been unable to determine the structure of the flavoprotein's reactive oxygen intermediate, a molecular complex that often forms halfway through important biochemical reactions. These intermediates possess high chemical potential energy, which is necessary to complete many critical but difficult-to-catalyze reactions in biology. Such intermediates typically have a lifetime of only a few milliseconds and are therefore very hard to observe using traditional synchrotron methods.
"Flavoproteins represent one of only a handful of ways that nature activates molecular oxygen, a process that's important for all life on the planet," said Brookhaven biophysicist Allen Orville. "We've determined structures of some oxygen intermediates involved in several important enzymes that assist in this process. But no one has ever seen an oxygen intermediate attached to the flavin. Until now."
As reported in the January 9, 2009, online edition of Biochemistry, Orville and colleagues from Georgia State University, Georgia Institute of Technology, and the University of Miami have used a new facility at Brookhaven's National Synchrotron Light Source (NSLS) to identify two possible oxygen intermediates in the flavoprotein, choline oxidase.
The researchers accomplished their work by combining two popular synchrotron techniques - x-ray diffraction and optical absorption spectroscopy - into one setup. By shining beams of powerful x-rays and visible light on the same region of the crystallized flavoprotein, two different but complementary sets of information are received. This allows the scientists to correlate the electronic structure of the enzyme - which gives details about chemical activities - with its three-dimensional atomic structure.
"The ability to collect multiple types of data from the same sample at the same time is a unique opportunity," Orville said. "It takes less time and it means you never have to move the sample and risk altering it in any way. It also removes many potential ambiguities that either technique alone cannot resolve."
To stabilize the flavoprotein intermediate, the researchers kept it at an extremely low temperature - about -280 degrees Fahrenheit. When exposed to the x-rays, the cold flavoprotein rapidly accepts electrons liberated in the sample by the x-ray beam. This starts the enzyme reaction, which progresses a bit further and then becomes trapped in its reactive intermediate state. Using the combined data, the group identified two possible intermediate structures. Further experiments will help determine which is the true intermediate.
Orville is installing additional complementary techniques at the NSLS. Planning also is underway for several beamlines with multiple complementary techniques at the National Synchrotron Light Source II, a new, proposed Brookhaven facility that will produce x-rays up to 10,000 times brighter than those at the NSLS. The hope is to provide a means for researchers to simultaneously obtain three or four different types of data from one sample.
This work was supported by DOE's Office of Biological and Environmental Research, the National Institutes of Health, the National Science Foundation, the American Chemical Society, the American Heart Association, Georgia State University, and the U.S. Department of Education. The operation of the NSLS is supported by the Office of Basic Energy Sciences within the DOE's Office of Science.
####
About Brookhaven National Laboratory
One of ten national laboratories overseen and primarily funded by the Office of Science of the U.S. Department of Energy (DOE), Brookhaven National Laboratory conducts research in the physical, biomedical, and environmental sciences, as well as in energy technologies and national security. Brookhaven Lab also builds and operates major scientific facilities available to university, industry and government researchers. Brookhaven is operated and managed for DOE’s Office of Science by Brookhaven Science Associates, a limited-liability company founded by Stony Brook University, the largest academic user of Laboratory facilities, and Battelle, a nonprofit, applied science and technology organization.
For more information, please click here
Contacts:
Kendra Snyder
(631) 344-8191
or
Mona S. Rowe
(631) 344-5056
Copyright © Brookhaven National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








