Home > Press > UO chemists create molecular 'claws' to trap arsenic atoms
Abstract:
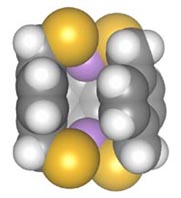
Chemists at the University of Oregon have hit upon a way to build a molecular "claw" that grabs onto arsenic and sequesters it.
UO chemists create molecular 'claws' to trap arsenic atoms
Discovery could eventually lead to improved treatments for arsenic poisoningEugene, OR – November 15, 2004
Chemists at the University of Oregon have hit upon a way to build a molecular "claw" that grabs onto arsenic and sequesters it.
The discovery is published in the Nov. 5 issue of Angewandte Chemie International Edition, a premier journal in the field of chemistry.
Since the article was written, the UO team has developed additional ways of capturing arsenic so that it cannot bond with other substances in a laboratory setting, according to Darren Johnson, an assistant professor of chemistry specializing in supramolecular and materials chemistry. Johnson, who joined the UO faculty in 2003, is also affiliated with the Oregon Nanoscience and Microtechnologies Institute (ONAMI).
The molecules developed by Johnson and one of his graduate students, Jake Vickaryous of Portland, are known as a chelators (pronounced "kee-lay-tor", from the Greek chele, meaning "crab claw"). A chelator's molecular configuration and binding sites enable it to trap and immobilize a heavy metal atom. In this case, a sulfur-based molecule was synthesized. In the presence of a toxic form of arsenic, three of these molecules bond with two arsenic atoms to create a triangular, pyramid-like molecular structure.
"By improving our understanding of these chemical interactions, we hope to develop more effective remediation agents-molecules that can do the work of rendering arsenic harmless," Johnson says.
Although they've demonstrated their new molecule can encapsulate arsenic in a laboratory setting, Johnson says, the challenge of treating poisoned individuals remains. The next step is to verify that the new molecule can render arsenic harmless without creating new problems in the human body.
"We're now trying to prove that our molecule wants arsenic more than things in your body want arsenic," says Johnson.
Numerous studies have linked consumption of minute amounts of arsenic in drinking water with higher incidences of lung, bladder, kidney and skin cancers, among other potentially fatal conditions. Arsenic is naturally abundant in the Earth's crust, and arsenic compounds are involved in some industrial applications.
The U.S. Environmental Protection Agency, in compliance with the Safe Water Drinking Act, currently requires that public water systems contain arsenic concentrations of less than 50 parts per billion (ppb). In 2006, this level is to be reduced to 10 ppb. This stricter standard has been endorsed by the World Health Organization since 1993.
Developing countries face serious problems due to arsenic-laced water sources but arsenic also is a problem in the United States. Roughly 10 percent of U.S. groundwater contains arsenic concentrations above 10 ppb. In Johnson's backyard, Oregon's bucolic Willamette Valley, more than 20 percent of wells have arsenic levels greater than 10 ppb. Of these, almost 10 percent exceed 50 ppb.
While they used computer-generated molecular models to predict many of the features they observed, Johnson says, the project also yielded some unexpected, and pleasant, surprises.
"We have stumbled upon some surprisingly stable self-assembled arsenic complexes. Someday, this approach may provide better agents for sensing and removing arsenic from the environment as well as the body," Johnson says.
Self-assembly refers to the ability of molecules to naturally join themselves together into larger structures due to the manners in which their geometric and binding structures complement one another. This feature, which is like a puzzle that puts itself together, is quite promising because it creates a final product that is more stable than the sum of its parts, Johnson explains.
In addition to modeled predictions, the structure of the molecule was confirmed using two primary methods. Nuclear magnetic resonance (NMR) spectroscopy uses the same principles that are the basis for magnetic resonance imaging (MRI), a commonly used medical scan of human tissue. The sample molecules are placed in a powerful magnetic field and are stimulated by specific patterns of radio waves. The patterns of energy that the molecules then release are interpreted to determine composition and structure. Another technique, X-ray diffraction, analyzes the scattering pattern of x-rays directed at a substance in order to characterize its atomic-scale structure.
Johnson, a UO assistant professor of chemistry, supervises the work of W. Jake Vickaryous (pronounced like the word "vicarious"), the UO doctoral degree candidate in chemistry who synthesized the molecule and is the lead author for the Angewandte Chemie article. Rainer Herges, the article's third co-author, is a professor at the Institut for Organische Chemie in Kiel, Germany, who produced the computer modeling studies for the project.
This phase of their work was funded by a UO research grant. In September, Vickaryous was awarded a National Science Foundation fellowship to support doctoral training at the interface of chemistry and physics. He will study new materials for electronics and optics through control of nanoscale structure.
The Oregon Nanoscience and Microtechnologies Institute is a collaboration involving Oregon's three public research universities-the University of Oregon, Oregon State University, Portland State University; the Pacific Northwest National Laboratory (Richland, Wash.); the state of Oregon; selected researchers from the Oregon Graduate Institute and the Oregon Health & Sciences University School of Dentistry; and the world-leading "Silicon Forest" high technology industry cluster of Oregon and southwest Washington.
Links:
Darren Johnson's home page
Angewandte Chemie (subscription needed)
Oregon Nanoscience and Microtechnologies Institute (ONAMI)
Institute of Organic Chemistry
Melody Ward Leslie
(541) 346-2060
mleslie@uoregon.edu
Source:
Darren Johnson
(541) 346-1695
dwj@uoregon.edu
Copyright © University of Oregon
If you have a comment, please us.
Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||