Home > Press > How to keep boron inside cells during radiotherapy: a simple novel approach to cancer treatment
 |
Abstract:
Boron neutron capture therapy (BNCT) is a technique in which p-boronophenylalanine (BPA) is transferred to cancer cells, and the boron in it undergoes nuclear fission reaction upon irradiation of thermal neutrons, releasing high energy particles that kill the cells. However, current technology allows only a short timeframe of BPA in the cell. Scientists at Tokyo Institute of Technology (Tokyo Tech), Kyoto University, and Innovation Center of NanoMedicine (iCONM) improved upon current BNCT by combining BPA with poly(vinyl alcohol) to produce the PVA-BPA complex, which has a longer cell retention time, enhancing the cancer-killing potential of BNCT.
How to keep boron inside cells during radiotherapy: a simple novel approach to cancer treatment
Tokyo, Japan | Posted on January 24th, 2020The growing knowledge about cancer biology has enhanced the development of many therapeutic strategies that involve selective targeting and killing of cancer cells. One such therapeutic option is boron neutron capture therapy (BNCT), a radiotherapy process involving transferring boron into cancer cells, and exposing it to neutron particles, causing it to undergo nuclear fission, which kills the cancer cells. If we can ensure that boron is present only in cancer cells, we can specifically destroy only cancer cells, without harming the normal cells of the body.
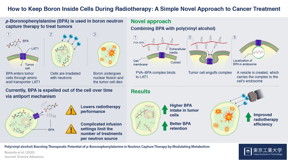
Scientists first discovered the way to induce this "selective uptake" using p-boronophenylalanine (BPA). BPA is a boron-containing compound with a phenylalanine structure. One of the factors that distinguishes cancer cells from normal cells is an overabundance of special structures called "LAT1 amino acid transporters", which recognize and allow the transport of phenylalanine into the cell. So, when BPA is present outside cancer cells, these transporters on the cell-surface allow it to enter cancer cells, permitting successful BNCT. BPA has long been considered the best drug available for BNCT.
However, BPA has a drawback; once BPA levels increase within the cancer cells, it is expelled back out via an "antiport" mechanism, making it challenging for BNCT to be effective in some cases. Thus, patients are required to receive a continuous infusion of BPA for 30–60 minutes, to maintain the necessary amount in the cells for the reaction to be successful. This also leaves the door open for human errors, such as the possibility of dislodging the needle during the process.
To overcome these drawbacks, scientists at Tokyo Tech, Kyoto University and iCONM led by Prof. Nobuhiro Nishiyama decided to explore other means of retaining boron inside cancer cells for longer periods. "We assumed," Prof. Nishiyama states, "that modulating the presence of BPA inside the cell will ensure that they are not sent back out via their antiport mechanism."
To examine this, they mixed a compound called poly(vinyl alcohol) (PVA) and BPA together, to form a PVA-BPA complex, and observed the internalization of this compound in cancer cells. They found that the addition of PVA did not affect the phenylalanine structure of BPA, allowing the LAT1 transporters to recognize the PVA-BPA complex. However, as this complex was too large to pass through the transporters, the LAT1 transporters instead engulfed the complex in organelles called endosomes and transported them into the cells. Since BPA was now safely located in endosomes, the cancer cell was not able to push it out immediately by the antiport mechanism. This ensured that boron remains in cells for a long enough time for the cancer cells to be effectively killed. This method was also tested in animal models, and anti-cancer activities of BNCT were found to be enhanced.
Addition of PVA thus presents an extremely simple solution to boost the therapeutic potential of BPA. "This technique is effortless," notes Prof. Nishiyama, "and offers a novel approach for drug delivery, focusing on the metabolic elimination processes of drugs. We will advance research on the PVA-BPA complex for clinical trials in cooperation with STELLA PHARMA CORPORATION who has conducted BPA clinical trials."
####
For more information, please click here
Contacts:
Assistant Professor Takahiro Nomoto
Institute of Innovative Research,,
Tokyo Institute of Technology
Tel +81-45-924 -5226
Professor Nobuhiro Nishiyama
Institute of Innovative Research,,
Tokyo Institute of Technology
Tel +81-45-924 -5240
Contact
Public Relations Section, Tokyo Institute of Technology
Tel +81-3-5734-2975
Copyright © Tokyo Institute of Technology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Cancer
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








