Home > Press > Neutrophil nanosponges soak up proteins that promote rheumatoid arthritis
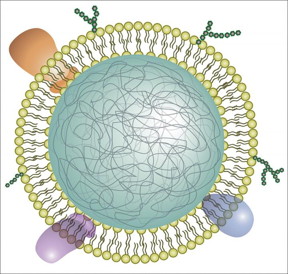 |
| Illustration of a neutrophil cell membrane-coated nanoparticle. CREDIT Qiangzhe Zhang/Nature Nanotechnology |
Abstract:
Engineers at the University of California San Diego have developed neutrophil "nanosponges" that can safely absorb and neutralize a variety of proteins that play a role in the progression of rheumatoid arthritis. Injections of these nanosponges effectively treated severe rheumatoid arthritis in two mouse models. Administering the nanosponges early on also prevented the disease from developing.
Neutrophil nanosponges soak up proteins that promote rheumatoid arthritis
San Diego, CA | Posted on September 3rd, 2018The work is published Sept. 3 in Nature Nanotechnology.
"Nanosponges are a new paradigm of treatment to block pathological molecules from triggering disease in the body," said senior author Liangfang Zhang, a nanoengineering professor at the UC San Diego Jacobs School of Engineering. "Rather than creating treatments to block a few specific types of pathological molecules, we are developing a platform that can block a broad spectrum of them, and this way we can treat and prevent disease more effectively and efficiently."
This work is one of the latest examples of therapeutic nanosponges developed by Zhang's lab. Zhang, who is affiliated with the Institute of Engineering in Medicine and Moores Cancer Center at UC San Diego, and his team previously developed red blood cell nanosponges to combat and prevent MRSA infections and macrophage nanosponges to treat and manage sepsis.
The new nanosponges are nanoparticles of biodegradable polymer coated with the cell membranes of neutrophils, a type of white blood cell.
Neutrophils are among the immune system's first responders against invading pathogens. They are also known to play a role in the development of rheumatoid arthritis, a chronic autoimmune disease that causes painful inflammation in the joints and can ultimately lead to damage of cartilage and bone tissue.
When rheumatoid arthritis develops, cells in the joints produce inflammatory proteins called cytokines. Release of cytokines signals neutrophils to enter the joints. Once there, cytokines bind to receptors on the neutrophil surfaces, activating them to release more cytokines, which in turn draws more neutrophils to the joints and so on.
The nanosponges essentially nip this inflammatory cascade in the bud. By acting as tiny neutrophil decoys, they intercept cytokines and stop them from signaling even more neutrophils to the joints, reducing inflammation and joint damage.
These nanosponges offer a promising alternative to current treatments for rheumatoid arthritis. Some monoclonal antibody drugs, for example, have helped patients manage symptoms of the disease, but they work by neutralizing only specific types of cytokines. This is not sufficient to treat the disease, said Zhang, because there are so many different types of cytokines and pathological molecules involved.
"Neutralizing just one or two types might not be as effective. So our approach is to take neutrophil cell membranes, which naturally have receptors to bind all these different types of cytokines, and use them to manage an entire population of inflammatory molecules," said Zhang.
"This strategy removes the need to identify specific cytokines or inflammatory signals in the process. Using entire neutrophil cell membranes, we're cutting off all these inflammatory signals at once," said first author Qiangzhe Zhang, a Ph.D. student in Professor Liangfang Zhang's research group at UC San Diego.
To make the neutrophil nanosponges, the researchers first developed a method to separate neutrophils from whole blood. They then processed the cells in a solution that causes them to swell and burst, leaving the membranes behind. The membranes were then broken up into much smaller pieces. Mixing them with ball-shaped nanoparticles made of biodegradable polymer fused the neutrophil cell membranes onto the nanoparticle surfaces.
"One of the major challenges of this work was streamlining this entire process, from isolating neutrophils from blood to removing the membranes, and making this process repeatable. We spent a lot of time figuring this out and eventually created a consistent neutrophil nanosponge production line," said Qiangzhe Zhang.
In mouse models of severe rheumatoid arthritis, injecting nanosponges in inflamed joints led to reduced swelling and protected cartilage from further damage. The nanosponges performed just as well as treatments in which mice were administered a high dose of monoclonal antibodies.
The nanosponges also worked as a preventive treatment when administered prior to inducing the disease in another group of mice.
Professor Liangfang Zhang cautions that the nanosponge treatment does not eliminate the disease. "We are basically able to manage the disease. It's not completely gone. But swelling is greatly reduced and cartilage damage is minimized," he said.
The team hopes to one day see their work in clinical trials.
###
This work is supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (grant HDTRA1-14-1-0064) and the National Science Foundation (grant DMR-1505699).
####
For more information, please click here
Contacts:
Liezel Labios
858-246-1124
Copyright © University of California, San Diego
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Military
![]() Quantum engineers ‘squeeze’ laser frequency combs to make more sensitive gas sensors January 17th, 2025
Quantum engineers ‘squeeze’ laser frequency combs to make more sensitive gas sensors January 17th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








