Home > Press > 'Decorated' stem cells could offer targeted heart repair
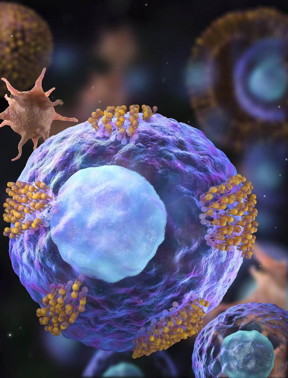 |
| Cardiac stem cells (magenta) are decorated with platelet vesicles (brown). CREDIT NC State University |
Abstract:
"Targeted repair of heart injury by stem cells fused with platelet nanovesicles"
DOI: 10.1038/s41551-017-0182-x
Authors: Junnan Tang, Teng Su, Ke Huang , Phuong-Uyen Dinh, Adam Vandergriff, Michael T. Hensley, Jhon Cores, Tyler Allen, Erin Sproul, Emily Mihalko, Ashely Brown, Laura Ruterbories, Alex Lynch, Zhen Gu, Ke Cheng, North Carolina State University; Junnan Tang, Deliang Shen, Jinying Zhang, First Affiliated Hospital of Zhengzhou University; Zegen Wang, Soochow University; Taosheng Li, Nagasaki University; Leonard J. Lobo, Thomas G. Caranasos, George A. Stouffer, Ashley Brown, Zhen Gu, Ke Cheng, University of North Carolina at Chapel Hill
Published: Nature Biomedical Engineering
Abstract:
Stem cell transplantation, as used clinically, suffers from low retention and engraftment of the transplanted cells. Inspired by the ability of platelets to recruit stem cells to sites of injury on blood vessels, we hypothesized that platelets might enhance the vascular delivery of cardiac stem cells (CSCs) to sites of myocardial infarction injury. Here, we show that CSCs with platelet nanovesicles fused onto their surface membranes express platelet surface markers that are associated with platelet adhesion to injury sites. We also find that the modified CSCs selectively bind collagen-coated surfaces and endothelium-denuded rat aortas, and that in rat and porcine models of acute myocardial infarction the modified CSCs increase retention in the heart and reduce infarct size. Platelet-nanovesicle-fused CSCs thus possess the natural targeting and repairing ability of their parental cell types. This stem cell manipulation approach is fast, straightforward and safe, does not require genetic alteration of the cells, and should be generalizable to multiple cell types.
'Decorated' stem cells could offer targeted heart repair
Raleigh, NC | Posted on January 11th, 2018"Platelets can home in on an injury site and stay there, and even in some cases recruit a body's own naturally occurring stem cells to the site, but they are a double-edged sword," says Cheng, associate professor of veterinary medicine and associate professor in the NC State/UNC Joint Department of Biomedical Engineering. "That's because once the platelets arrive at the site of injury, they trigger the coagulation processes that cause clotting. In a heart-attack injury, blood clots are the last thing that you want."
Cheng and his associates wondered if it would be possible to co-opt a platelet's ability to locate and stick to an injury site without inducing clotting. They found that adhesion molecules (a group of glycoproteins) located on the platelet's surface were responsible for its ability to find and bind to an injury. So the team created platelet nanovesicles from these molecules, and then decorated the surface of cardiac stem cells with the nanovesicles,
"The nanovesicle is like the platelet's coat," Cheng says. "There isn't any internal cellular machinery that could activate clotting. When you place the nanovesicle on the stem cell, it's like giving the stem cell a tiny GPS that helps it locate the injury so it can do its repair work without any of the side effects associated with live platelets."
In a proof-of-concept study involving a rat model of myocardial infarction, twice as many platelet nanovesicle decorated cardiac stem cells, or PNV-CSCs, were retained in the heart than non-decorated cardiac stem cells. The rodents were monitored for four weeks. Overall, the rats in the PNV-CSC group showed nearly 20 percent or higher cardiac function than the control CSC group.
A small pilot study in a pig model also demonstrated higher rates of stem cell retention with PNV-CSCs, though the team did not perform functional studies. A future follow-up study is planned.
"Platelet nanovesicles do not affect the performance of the cardiac stem cells, and are free from any negative side effects," Cheng says. "Hopefully we will be able to use this approach to improve cardiac stem cell therapy in clinical trials in the future."
###
The work appears in Nature Biomedical Engineering and was funded by the National Institutes of Health, the NC State University Chancellor's Excellence Fund, and the UNC General Assembly Research Opportunities Initiative Grant. Cheng is corresponding author, as well as associate director of the Comparative Medicine Institute and leader of the BioTherapeutics Laboratory. Dr. George (Rick) Stouffer, chief of cardiology at the University of North Carolina Chapel-Hill, also contributed to the work.
####
For more information, please click here
Contacts:
Tracey Peake
919-515-6142
Copyright © North Carolina State University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








