Home > Press > Multicolor super resolution imaging: A method to monitor dynamic protein binding at subsecond timescales
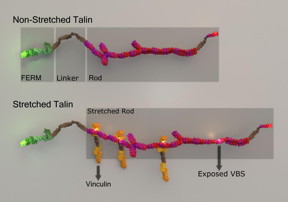 |
| Talin stretching and stretch-induced vinculin binding. CREDIT: Mechanobiology Institute, National University of Singapore |
Abstract:
Researchers from the Mechanobiology Institute (MBI) at the National University of Singapore have developed a new method, using super-resolution microscopy, to determine the length of stretched proteins in living cells, and monitor the dynamic binding of proteins, at sub-second timescales. This study was published in Nano Letters in May 2016.
Multicolor super resolution imaging: A method to monitor dynamic protein binding at subsecond timescales
Singapore | Posted on June 19th, 2016Monitoring force-induced talin stretching and the dynamic binding of vinculin to talin
Cells are constantly exposed to mechanical forces. These signals influence cellular decision making by providing information cells need to determine how much of a particular protein to produce, when a specific gene should be expressed, or even whether a cell should move or remain where it is. Such information is crucial, for example, in maintaining the health, integrity and repair of tissues as we age. A clear example of when cells are exposed to forces is when we walk. Stretching or pulling forces are generated within our muscles, and these are passed through the muscle to connective tissue and bone. Although this information is generated at a tissue level, it converges on single cells within those tissues, and is detected and measured by subcellular, protein based, machines.
To measure the forces applied to a cell, specialized proteins may be deformed. A common way that this occurs is when a protein is stretched, just like how an elastic band stretches when subjected to pulling forces. Stretching of proteins can expose regions within them that are otherwise hidden. These regions can serve as docking sites for the attachment of other proteins. This leads to a snowball effect, wherein more and more proteins are able to bind, and larger molecular complexes or machines form to mediate a specific cellular function. This phenomena was recently explored by MBI Director, Professor Michael Sheetz, Senior Research Fellow Dr Felix Margadant and PhD student Ms Xian Hu (Edna), in work focused on characterizing the stretching of a force-sensing protein known as talin, and establishing the effect it has on the binding of another protein called vinculin.
Although several studies have shown the force-induced stretching of talin and talin-vinculin binding in vitro, simultaneous visualization of both these events and their correlation to specific cellular functions was not previously possible in living cells due to the rapid time scales at which they occur. Also, carrying out multicolor super resolution imaging in living cells is still very difficult. To overcome these challenges, Prof Sheetz and Ms Hu developed a novel, and highly advanced super-resolution imaging method, that allowed them to simultaneously monitor talin length in living cells, as well as the dynamics of vinculin binding, at single molecule level and millisecond timescale.
By attaching different fluorescent molecules (GFP and mCherry), to each end of the talin and a third fluorophore (Atto655) to vinculin, the researchers could monitor the precise subcellular location of each protein, and confirm that when talin was being stretched, vinculin bound to newly exposed sites. Interestingly, their findings often revealed clustered binding, with five or more vinculin molecules binding to talin in one second. Moreover, the binding of the first few vinculins seemed to energetically favor the successive binding of more vinculin molecules. Correlating vinculin binding dynamics with the amount of talin stretching, the researchers noted that maximum vinculin binding occurred at one specific end of talin (the N-terminal region), when talin was stretched to approximately 180 nm.
Understanding how talin and vinculin respond to stretching forces is crucial to understanding how cells respond to forces in our bodies. In this case, both proteins are found in larger molecular machinery called focal adhesions, which physically connect the interior of a cell with the material that is surrounding the cell, the extracellular matrix. Focal adhesions primarily function as signal relaying centers, and the information they transfer can induce cell growth and cell movement. When this signal processing is disrupted, or is not regulated, disease states arise and the body's ability to heal wounds, or maintain tissue integrity as we age becomes impaired.
Although important to facilitating these wider cellular and tissue processes, the talin-vinculin interaction is just one of many protein interactions to respond to force. It is hoped that this newly described method will pave the way for researchers to dissect other protein interactions, both within focal adhesions, and in other molecular machines, to improve our understanding of the many force-driven cellular processes that arise during development and continue through to aging.
####
For more information, please click here
Contacts:
Amal Naquiah
65-651-65125
Copyright © National University of Singapore (NUS)
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Life Extension/Cryonics
![]() Ageing can drive progress: Population ageing is likely to boost medicine, nanotechnology and robotics, but increase political risks July 27th, 2016
Ageing can drive progress: Population ageing is likely to boost medicine, nanotechnology and robotics, but increase political risks July 27th, 2016
![]() Preventing protein unfolding: Polymers can reinforce proteins under mechanical forces February 27th, 2016
Preventing protein unfolding: Polymers can reinforce proteins under mechanical forces February 27th, 2016
![]() Lifeboat Foundation launches 3 books December 16th, 2015
Lifeboat Foundation launches 3 books December 16th, 2015
![]() Hopes of improved brain implants October 1st, 2015
Hopes of improved brain implants October 1st, 2015
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








