Home > Press > Acoustic tweezers moves cells in three dimensions, builds structures
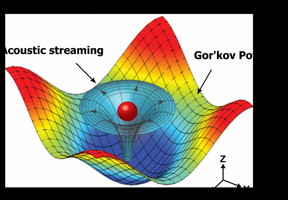 |
| Numerical simulation results mapping the acoustic field around a particle that shows the physical operating principle for the 3-D acoustic tweezers. The 3-D trapping node in the microfluidic chamber is created by two superimposed, orthogonal, standing surface acoustic waves and the induced acoustic streaming. CREDIT: Tony Jun Huang, Penn State |
Abstract:
Acoustic tweezers that can move single cells in three dimensions using surface acoustic waves without touching, deforming or labeling the cells are possible, according to a team of engineers.
Acoustic tweezers moves cells in three dimensions, builds structures
University Park, PA | Posted on January 26th, 2016"In this application we use surface acoustic waves to create nodes where cells or microparticles are trapped," said Tony Jun Huang, professor and The Huck Distinguished Chair in Bioengineering Science and Mechanics. "We can then move the cell or particle in three dimensions to create structures in two or three dimensions."
The trapping nodes are formed by two sets of surface-acoustic-wave generators. When the sound waves from opposite sides meet, they create pressure that catches and positions the particle or cell. Moving the location where the sound waves meet moves the location of the cell or particle. These standing-wave shifts manipulate the tiny objects in two dimensions. The amplitude of the acoustic vibrations controls the movement in the third dimension. The researchers report their work in today's (Jan. 25) issue of the Proceedings of the National Academy of Sciences.
"The results presented in this paper provide a unique pathway to manipulate biological cells, accurately and in three dimensions, without the need for any invasive contact, tagging, or biochemical labeling," said Subra Suresh, president, Carnegie Mellon University and part of the research team. "This approach could lead to new possibilities for research and applications in such areas as regenerative medicine, neuroscience, tissue engineering, biomanufacturing, and cancer metastasis."
The research team not only created a 3-D tweezers, but they also modeled bioprinting with this device and used the device to pick up, translate and print single cells and cell assemblies, creating 2-D and 3-D structures in a precise, noninvasive manner. They demonstrated this ability by capturing a single suspended mouse fibroblast and moving it to a targeted location in the microfluidic chamber.
Bioprinting to recreate biological materials must include a way to preserve cell-to-cell communications and cell-environment interactions. While the device is not a 3-D printer in the conventional sense, it can move specific cells and particles to specified places and attach them wherever they belong in a functional way.
"Adding a third dimension for precisely manipulating single cells for bioprinting further advances acoustic tweezers technology," said Ming Dao, director, Nanomechanics Lab, Massachusetts Institute of Technology. "The accompanying modeling provides solutions for cell manipulation, enabling validation of the method as well as possible system optimization."
The third dimension achieved with this device relies on acoustic streaming, a type of fluidic motion induced by a standing acoustic wave. By manipulating the acoustic wave, the researchers could position the trapped particle or cell wherever they wanted it within the vertical confines of the enclosed fluid.
"3-D acoustic tweezers can pattern cells with control over the number of cells, cell spacing and the confined geometry, which may offer a unique way to print neuron cells to create artificial neural networks for neuron science applications or regenerative neuron medicine," said Huang.
The current device can place a cell or particle with 1 micrometer accuracy horizontally and with 2 micrometer accuracy vertically. The researchers moved a 10 micrometer particle at an average speed of about 2.5 micrometers per second and could place cells in several seconds to a few minutes depending on the distance.
Because the acoustic wavelength and input power are instantaneously tunable during experiments, the placement accuracy is only limited by the resolution of the device setup, according to the researchers.
###
Also working on this project were Feng Guo, Peng Li and James Lata, postdoctoral Fellows in engineering science and mechanics; Zhangming Mao and Yuchao Chen, graduate students in engineering science and mechanics; Zhiwei Xie, former postdoctoral Fellow in biomedical engineering; and Jian Yang, professor of biomedical engineering, all at Penn State.
The National Institutes of Health and the National Science Foundation suported this work.
####
For more information, please click here
Contacts:
A'ndrea Elyse Messer
814-865-9481
Copyright © Penn State
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








