Home > Press > New nanoscopic tools to study ligand-binding of receptors and quantifying two ligand-binding sites while imaging membrane receptors
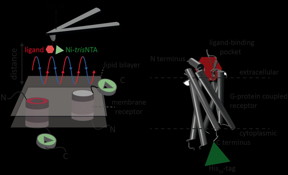 |
| A new high resolution method developed by an international team of scientists including Robert Tampé and Ralph Wieneke from Goethe University Frankfurt now allows for the first time precise identification and quantification of interactions of a receptor with two ligands simultaneously. CREDIT: GU |
Abstract:
Signalling processes in organisms are governed by specific extracellular and intracellular interactions and involve hundreds of different functionally highly versatile receptors situated in cell membranes. For scientists wishing to understand signalling processes the situation is made more complex by the receptors not only being unevenly distributed and often able to bind more than one ligand but also by the same type of receptor being able to bind a ligand strongly, weakly or not all. New methods that allow precise quantifications of such complex interactions are urgently required.
New nanoscopic tools to study ligand-binding of receptors and quantifying two ligand-binding sites while imaging membrane receptors
Frankfurt, Germany | Posted on November 18th, 2015A new high resolution method developed by an international team of scientists including Robert Tampé and Ralph Wieneke from Goethe University Frankfurt now allows for the first time precise identification and quantification of interactions of a receptor with two ligands simultaneously. The new method has been published in the latest edition of the journal Nature Communications.
Atomic force microscopy (AFM) is a powerful technique for nanoscale characterization of surfaces. It makes use of a cantilever with an extremely fine tip. Force-distance curve-based atomic force microscopy (FD-based AFM) combines high-resolution imaging and single-molecule force spectroscopy. In studies using biological samples, the AFM tip approaches and retracts from the sample for each pixel. FD-based AFM methods use different coatings of the AFM tip as a toolbox and such methods have made impressive progress in recent years. For the detection of specific binding sites FD-based AFM requires tethering of a ligand to the AFM tip. While contouring protein complexes in a membrane such functionalized AFM tips can then measure the interactions of the tethered ligand to the protein. It had not been possible to image single membrane receptors and simultaneously detect their interactions with more than one ligand, but the new method has overcome this hurdle.
For their proof of principle the scientists used the human protease-activated receptor 1 (PAR1), one of the large family of G-protein-coupled membrane receptors. GPCRs mediate most cellular responses to hormones and neurotransmitters, as well as being responsible for vision, olfaction and taste. GPCRs can coexist in different functional states in the cell membrane and can bind various ligands at different strength or affinity. The GPCR PAR1 is activated by the coagulation protease thrombin which triggers signalling cascades to initiate cellular responses that help orchestrate haemostasis, thrombosis, inflammation and possibly also tissue repair. With the aid of their new FD-based AFM method human PAR1 in proteoliposomes could be imaged while simultaneously detecting extracellular and intracellular interactions of PAR1 with two ligands. The surface chemistry and nanoscopic method developed are applicable to a range of biological systems in vitro and in vivo.
####
For more information, please click here
Contacts:
Robert Tampé
49-069-798-29475
Copyright © Goethe University Frankfurt
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








