Home > Press > Targeted nanoparticles can overcome drug resistance in trypanosomes: A high-tech approach to combat sleeping sickness and potentially other neglected diseases
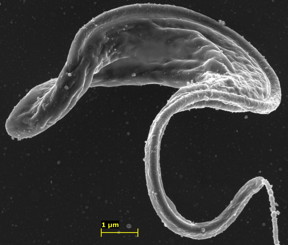 |
| This is the parasite Trypanosoma brucei with targeted nanoparticles adhered to its surface. CREDIT: Garcia-Salcedo et al, CC-BY |
Abstract:
Sleeping sickness, or African trypanosomiasis, is caused by trypanosome parasites transmitted by tsetse flies and threatens millions of people in sub-Saharan Africa. The disease is considered fatal if untreated, but as it affects mostly poor people in low-income countries, treatment options are limited. The existing drugs have serious side effects, and the parasites are developing resistance. A study published on June 25th in PLOS Pathogens reports a new way to circumvent drug resistance and lower the curative dose by delivering existing drugs directly into the parasite, a high-tech approach with potential applications to other infectious diseases.
Targeted nanoparticles can overcome drug resistance in trypanosomes: A high-tech approach to combat sleeping sickness and potentially other neglected diseases
San Francisco, CA | Posted on June 26th, 2015Current treatment of sleeping sickness relies primarily on four drugs. Three of these drugs get into the interior of the parasite cells via the trypanosome's transport proteins that normally supply the parasite with nutrients, and drug resistance is caused by mutations that cripple these transporters. Jose Garcia-Salcedo, from the Instituto de Investigacion Biosanitaria in Granada, Spain, and colleagues reasoned that using an alternative way to get the drugs into parasite cells would circumvent resistance.
The researchers developed a drug carrier that consists of polymeric nanoparticles coated with specialized antibodies that target a small conserved (i.e., invariable) part of the parasite surface. (Much of the trypanosome surface is highly variable, which is why the chances of developing an effective vaccine have been deemed low.) They show that this new formulation reduces the minimal curative dose in a disease model, based on infections in mice, by 100-fold and, most importantly, circumvents drug resistance in a cell line that is resistant as a result of mutations in the transporter that mediates drug uptake.
The authors conclude "in summary, the development of chitosan nanoparticles loaded with current trypanocidal drugs coated by a specific nanobody against trypanosomes can reduce the minimal curative dose of these drugs, enhancing their efficacy, minimizing the toxicity and circumventing resistance mechanisms caused by mutations in surface transporters."
The implication of this proof-of-concept study of a novel technology for reversing transporter-related drug resistance, they say, "is not limited to a single nanobody used to demonstrate the technology, nor to a single drug, nor indeed to trypanosomiasis." "With a key challenge being that resistance to drugs is spreading faster than new drugs are being developed and approved", they suggest that "the use of encapsulated, nanobody-targeted drugs as described here has the potential to reverse resistance to many first-line treatments."
Authors and Affiliations:
Juan D. Unciti-Broceta, Instituto de Investigación Biosanitaria ibs.GRANADA, Spain; Instituto de Parasitología y Biomedicina "López-Neyra" (IPBLN-CSIC), Spain; Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica (GENYO), Spain
José L. Arias, Universidad de Granada, Spain
José Maceira, Instituto de Investigación Biosanitaria ibs.GRANADA, Spain; Instituto de Parasitología y Biomedicina "López-Neyra" (IPBLN-CSIC), Spain; Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica (GENYO), Spain
Miguel Soriano, Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica (GENYO), Spain; Universidad de Almería, Spain
Matilde Ortiz-González, Instituto de Investigación Biosanitaria ibs.GRANADA, Spain; Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica (GENYO), Spain
José Hernández-Quero, Instituto de Investigación Biosanitaria ibs.GRANADA, Hospitales Universitarios de Granada/Universidad de Granada, Spain
Manuel Muñóz-Torres, Instituto de Investigación Biosanitaria ibs.GRANADA, Hospitales Universitarios de Granada/Universidad de Granada, Spain
Harry P. de Koning, Institute of Infection, Immunity and Inflammation, University of Glasgow, UK
Stefan Magez, Vrije Universiteit Brussel, Belgium
José A. Garcia-Salcedo, Instituto de Investigación Biosanitaria ibs.GRANADA, Spain; Instituto de Parasitología y Biomedicina "López-Neyra" (IPBLN-CSIC), Spain; Centro Pfizer-Universidad de Granada-Junta de Andalucía
de Genómica e Investigación Oncológica (GENYO), Spain
Funding: JAGS was funded by the European Union, grant FP7-HEALTH-2007-B-2.3.4-1.223048, NANOTRYP and Ministerio de Economía y Competitividad, Spain Plan Nacional de Investigación grant SAF2011- 30528. JLA was funded by Instituto de Salud Carlos III, Spain, grant FIS. 11/02571. HPdK was supported by a grant from the Medical Research Council (84733). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests: The authors have declared that no competing interests exist.
Citation: Unciti-Broceta JD, Arias JL, Maceira J, Soriano M, Ortiz-González M, Hernández-Quero J, et al. (2015) Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis. PLoS Pathog 11(6): e1004942. doi:10.1371/journal.ppat.1004942
####
For more information, please click here
Contacts:
Jose Garcia-Salcedo
34-679-187-751
Stefan Magez
phone: +32.474.05.93.62
cell: +32.474.05.93.62
Please contact if you would like more information.
Copyright © PLOS
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








