Home > Press > Device extracts rare tumor cells using sound: Microfluidic chip developed by CMU President Suresh and collaborators uses acoustic waves to separate circulating tumor cells from blood cells
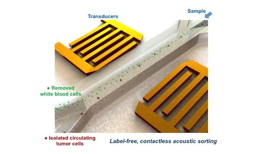 |
| This schematic illustration shows the high-throughput device for separating cancer cells from white blood cells. CREDIT: Carnegie Mellon/MIT/Penn State |
Abstract:
-A simple blood test may one day replace invasive biopsies thanks to a new device that uses sound waves to separate blood-borne cancer cells from white blood cells.
Device extracts rare tumor cells using sound: Microfluidic chip developed by CMU President Suresh and collaborators uses acoustic waves to separate circulating tumor cells from blood cells
Pittsburgh, PA | Posted on April 7th, 2015Carnegie Mellon University President Subra Suresh and fellow researchers from the Massachusetts Institute of Technology and The Pennsylvania State University report the latest advancement that brings their device one step closer to clinical use in a paper published this week in the online early edition of the Proceedings of the National Academy of Sciences (PNAS).
Earlier attempts to develop such a device, while promising, proved to be far too slow for clinical use. But in the new paper, the researchers report the development and validation of a microfluidic chip that uses sound waves to separate circulating tumor cells (CTC) from white blood cells, to be up to 20 times faster than prior attempts. This enables the use of the device for basic research and makes the idea that clinicians could remove intact circulating tumor cells (CTCs) from a standard blood draw closer to becoming a reality.
Additionally, the researchers tested their device, for which a patent application has been filed, using patient samples for the first time. These tests proved that their method for separating CTCs from cancer cells is as effective as the current FDA-approved technique.
"Using computer modeling, we were able to significantly improve the chip's throughput. With further refinements, this device could enhance our ability to diagnose and treat cancer," said Suresh, a co-author of the study who holds faculty appointments in CMU's College of Engineering, School of Computer Science and Heinz College. "The current gold-standard for finding CTCs requires scientists to tag the cells using antibodies. Our technique has the added advantage of being label-free, without the need for any tagging that could chemically alter the cells. Our new approach would allow scientists and clinicians to gain more information on cell pathology and cancer metastasis than is currently possible."
When a physician wants to determine whether or not a tumor is cancerous, or learn more about a patient's cancer -- for instance, whether or not a tumor has metastasized, or if a tumor is genetically predisposed to respond to a certain therapy -- a sampling of cells is removed from the tumor through a biopsy. Depending on the location of the tumor, a biopsy can be painful and invasive.
CTCs offer the promise of a much less invasive option, often referred to as a "liquid biopsy." CTCs are cells that have been shed from a primary tumor and circulate through the body via the blood stream. They are of great interest to cancer researchers and clinicians because they offer a way to gather cancer cells through a routine blood draw rather than a biopsy. CTCs can provide more information about metastasis, response to treatment and the genetic nature of a patient's cancer than cells taken directly from a tumor.
In many cases, CTCs are too rare to be detectable because there might be only one CTC among hundreds of thousands of white blood cells. Currently, most researchers find and isolate the traveling tumor cells either by using fluorescence and magnetic techniques or by mechanical means. Some of these methods require the cancer cells to be tagged using antibodies, which can alter the cell's genetic and physical make-up. Other research groups have developed ways to remove CTCs using strong mechanical forces, which also can damage the cells. While all of these techniques allow researchers to count the number of CTCs in a blood sample for possible cancer diagnosis, researchers often can't use the altered cells to reliably perform any additional functional tests.
The new device could help to solve this problem. It doesn't require the use of any damaging tags. It uses only a gentle mechanical force created by sound waves, allowing scientists to recover whole, unaltered CTCs that are ready for further testing. The authors also have demonstrated that, at the cell level, the device preserves the integrity of the separated CTCs, somewhat similar to the gentle way in which ultrasound has long been used in medical imaging and diagnostics.
To test a blood sample using the device, the researchers remove the red blood cells from the sample, and introduce the remaining blood product into a channel in the dime-sized chip. As the sample travels along the channel, it passes through sound waves that have been angled across the channel. By angling the sound waves, the researchers have created a gauntlet of pressure nodes that push the cells away from the center of the channel. Since cancer cells have different size and compressibility than normal white blood cells, they are propelled at different trajectories by the sound waves. The researchers were able to establish what path each cell type would take, and created two channels along those paths to collect the separated cells.
In previous versions of the device, the throughput rate was too slow for clinical use -- it would have taken between 30 and 60 hours to separate the cells in one vial of blood (approximately 5 milliliters). The researchers used computer modeling and parametric numerical simulations to find ways to alter the device to make it more efficient. From models, they were able to come up with an optimized design for the geometry of the channels on the chip, and tilt angles of and width of the acoustic transducers.
They took the information generated by the simulations and created new sample prototypes. The most efficient prototype processed a sample 20 times faster than their original prototype, taking only approximately five hours to process a 5-milliliter sample.
The researchers used this faster version of the chip to test blood samples from three women with diagnosed breast cancer. In terms of counting CTCs, their results were found to be consistent with previous tests performed using the traditional FDA-approved CellSearch ® cell-tagging method.
Furthermore, in one of the samples, they found only one CTC. On further consultation with that patient's doctor, they found that the patient was responding well to treatment, further demonstrating the device's promise for detecting cancer and metastasis, and monitoring the effectiveness of treatment.
The increased speed of the device makes it ready to use for laboratory research, but researchers warn that it's still too slow for broad clinical use. They plan to continue to refine the device to make it more suitable for separating CTCs from a vial of blood in less than 30 minutes, which will make it more amenable to commercialization. They also hope to develop a version of the device that can be used with whole blood samples, eliminating the need to remove the red blood cells.
President Suresh is in a rare group of academic leaders who have maintained a strong scholarly presence in research. His individual academic achievements have been globally recognized and have continued in addition to his leadership accomplishments throughout his major responsibilities as dean of the school of engineering at MIT, as director of the National Science Foundation, and now as president of Carnegie Mellon. Since accepting the presidency of CMU, Suresh has published about a dozen articles in leading scientific journals and has filed several CMU patents.
Suresh said that while the duties of the presidency at CMU are his top priorities and focus, he intends to continue, to the extent possible, connections to research and to the mentoring and training of young scholars.
"A university president is first and foremost, a member and representative of the faculty," Suresh said. "Leadership and scholarship should not be mutually exclusive. I think that it is important for those of us in leadership roles to remain close to our academic roots and to the heart of the academic enterprise, and to continue to remind ourselves why we are in academia. In that context, the current work has been very gratifying for me personally."
###
Co-authors of the study, whose research was supported by the National Institutes of Health (1 R01 GM112048-01A1, 1 R33 EB019785-01), the Penn State Center for Nano Scale Science and the National Science Foundation, include: Peng Li, Zhanming Mao, Yuchao Chen, Po-Hsun Huang and Tony Jun Huang of The Pennsylvania State University; Zhangli Peng (now at the University of Notre Dame) and Ming Dao of the Massachusetts Institute of Technology; and Lanlan Zhou, Cristina I. Truica, Joseph H. Drabick, Wafik S. El-Deiry of the Penn State Hershey Cancer Institute.
####
About Carnegie Mellon University
Carnegie Mellon is a private, internationally ranked research university with programs in areas ranging from science, technology and business, to public policy, the humanities and the arts. More than 13,000 students in the university's seven schools and colleges benefit from a small student-to-faculty ratio and an education characterized by its focus on creating and implementing solutions for real problems, interdisciplinary collaboration and innovation.
For more information, please click here
Contacts:
Jocelyn Duffy
412-268-9982
Copyright © Carnegie Mellon University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Microfluidics/Nanofluidics
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
Cancer
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Research partnerships
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








