Home > Press > Chromium-centered cycloparaphenylene rings for making functionalized nanocarbons
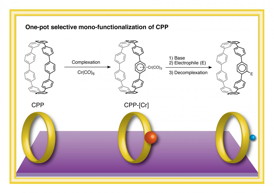 |
| This image shows a one-pot selective monofunctionalization of CPP via a chromium complex. CREDIT: ITbM, Nagoya University |
Abstract:
A team of chemists at Nagoya University has synthesized novel transition metal-complexed cycloparaphenylenes (CPPs) that enable selective monofunctionalization of CPPs for the first time, opening doors to the construction of unprecedented nanocarbons.
Chromium-centered cycloparaphenylene rings for making functionalized nanocarbons
Nagoya, Japan | Posted on January 26th, 2015Professor Kenichiro Itami, Yasutomo Segawa and Natsumi Kubota of the JST-ERATO Itami Molecular Nanocarbon Project and the Institute of Transformative Bio-Molecules (ITbM), Nagoya University have synthesized novel cycloparaphenylene (CPP) chromium complexes and demonstrated their utility in obtaining monofunctionalized CPPs, which could become useful precursors for making carbon nanotubes with unprecedented structures. CPPs consist of a chain of benzene rings and are the shortest segment of carbon nanotubes. Since their first synthesis and isolation in 2008, CPPs have attracted wide attention in the fields of materials science and supramolecular chemistry. Applying the basic concepts of chromium arene chemistry, Itami and his coworkers have performed the first selective installation of a functional group on CPP, which has previously been difficult due to multiple reactive arene sites on the CPP ring. By being able to selectively install and tune the functional groups on CPPs, it is envisaged that carbon nanotubes with new properties can be constructed by this method. The study, published online on January 12, 2015 in the Journal of the American Chemical Society, illustrates the first synthesis, isolation and analysis of a CPP chromium complex, which enables a one-pot access to monofunctionalized CPPs. This outcome is believed to be a significant advance in the fields of both CPP chemistry and organometallic chemistry.
Arenes are known to coordinate to transition metals and the corresponding metal complexes exhibit different reactivities relative to the free arene. CPPs, which consist of a chain of arenes, also reacted with chromium carbonyl to successfully generate the first chromium complex of CPP. Interestingly, the main product was a CPP with one chromium moiety complexed to one arene on the outer side of the ring, as confirmed by 1H NMR (nuclear magnetic resonance) spectroscopy, high-resolution mass spectrometry and X-ray crystallography.
"Chromium arene chemistry is a well-established area and we decided to apply this organometallic method to synthesize the first CPP chromium complex," says Itami, the Director of the JST-ERATO project and the Institute of Transformative Bio-Molecules. "As CPPs have a number of arene rings, we initially expected that chromium would form a complex with each arene ring," says Segawa, a group leader of the JST-ERATO project. "However, we were surprised to see that CPP reacted with chromium in a 1:1 ratio in all the conditions that we tried. Simulation of the molecular structure suggested that the first equivalent of chromium complexed to CPP lowers its reactivity, thus preventing the reaction with a second chromium moiety."
Upon finding that a monometallic CPP complex could be obtained, Itami's team explored the possibility of obtaining monofunctionalized CPPs from this complex. Itami and Segawa describe the steps in achieving this. "This was not an easy task as chromium arene complexes are usually air and light sensitive, and CPP chromium complexes were no exception. But Natsumi worked persistently to obtain a pure crystal of the first CPP chromium complex," says Itami. "We then performed the subsequent reactions in one-pot, to synthesize monofunctionalized CPPs after addition of base/electrophiles and removal of the metal from the CPP chromium complex," says Segawa.
Selective monofunctionalizations of CPPs i.e. installation of one functional group at a single position on the arene ring, are difficult to achieve as all carbon-hydrogen bonds on the arene rings are chemically equivalent. Direct functionalization of metal-free CPPs usually leads to multiple substitutions on the arene rings in an uncontrolled manner. Despite CPPs being desirable components for carbon nanotubes, there has been no efficient method to obtain directly functionalized CPPs up to now.
"We were pleased to see that a functional group could be selectively installed on one arene ring via chromium coordination of CPPs," says Segawa. "As electrophiles, we utilized silyl, boryl and ester groups, which act as handles that can be easily transformed to other useful functionalities," he continues. Itami says, "We hope that this new approach evolves to become a valuable method to construct carbon nanotubes with unique structures and properties."
###
This article "η6-Cycloparaphenylene Transition Metal Complexes: Synthesis, Structure, Photophysical Properties, and Application to the Selective Monofunctionalization of Cycloparaphenylenes" by Natsumi Kubota, Yasutomo Segawa, and Kenichiro Itami is published online on January 12, 2015 in the Journal of the American Chemical Society.
DOI: 10.1021/ja512271p
####
About ITbM, Nagoya University
The World Premier International Research Center Initiative (WPI) for the Institute of Transformative Bio-Molecules (ITbM) at Nagoya University in Japan is committed to advance the integration of synthetic chemistry, plant/animal biology and theoretical science, all of which are traditionally strong fields in the university. As part of the Japanese science ministry's MEXT program, ITbM aims to develop transformative bio-molecules, innovative functional molecules capable of bringing about fundamental change to biological science and technology. Research at ITbM is carried out in a "Mix-Lab" style, where international young researchers from multidisciplinary fields work together side-by-side in the same lab. Through these endeavors, ITbM will create "transformative bio-molecules" that will dramatically change the way of research in chemistry, biology and other related fields to solve urgent problems, such as environmental issues, food production and medical technology that have a significant impact on the society.
About JST-ERATO Itami Molecular Nanocarbon Project
This project entails the design and synthesis of as-yet largely unexplored nanocarbons as structurally well-defined molecules, and the development of novel, highly functional materials based on these nanocarbons. Through the combination of chemical and physical methods, the project aims to achieve the controlled synthesis of well-defined uniquely structured nanocarbon materials. Interdisciplinary research is conducted to encompass the control of molecular arrangement and orientation, structural and functional analysis, and applications in devices and biology.
About JST-ERATO
ERATO (The Exploratory Research for Advanced Technology), one of the Strategic Basic Research Program, aims to form a headstream of science and technology, and ultimately contribute to science, technology, and innovation that will change society and the economy in the future. In ERATO, a Research Director, a principal investigator of ERATO research project, establishes a new research base in Japan and recruits young researchers to implement his or her challenging research project within a limited time frame.
For more information, please click here
Contacts:
Author Contact
Professor Kenichiro Itami
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL/FAX: +81-52-788-6098
Media Contact
Dr. Ayako Miyazaki
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
Furo-Cho, Chikusa-ku, Nagoya 464-8601, Japan
TEL: +81-52-789-4999 FAX: +81-52-789-3240
Nagoya University Public Relations Office
TEL: +81-52-789-2016 FAX: +81-52-788-6272
Copyright © ITbM, Nagoya University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanotubes/Buckyballs/Fullerenes/Nanorods/Nanostrings/Nanosheets
![]() Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
Tiny nanosheets, big leap: A new sensor detects ethanol at ultra-low levels January 30th, 2026
![]() Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
Enhancing power factor of p- and n-type single-walled carbon nanotubes April 25th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Innovative biomimetic superhydrophobic coating combines repair and buffering properties for superior anti-erosion December 13th, 2024
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








