Home > Press > Tokyo Institute of Technology research: Protein-engineered cages aid studies of cell functions
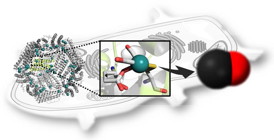 |
| Protein engineered ferritin cages provide capsules for carbon monoxide releasing molecules that allow slow release for enhanced activation of nuclear factors inside the cells, according to research at Tokyo Institute of Technology and Nagoya University. |
Abstract:
Researchers at Tokyo Institute of Technology have engineered protein cages for delivering an important signalling molecule, carbon monoxide, into cells.
Tokyo Institute of Technology research: Protein-engineered cages aid studies of cell functions
Tokyo, Japan | Posted on November 19th, 2014Carbon monoxide (CO) plays an important role in cell functions, by signalling responses that counteract inflammation, and cell growth and death. As a result, researchers have been in pursuit of molecules that release CO into cells in order to study biological responses. Now Takafumi Ueno and colleagues in Japan report in the Journal of the American Chemistry Society how they have developed a protein cage that overcomes the limitations of previously reported CO releasing molecules.
The CO-releasing molecules developed so far, such as ruthenium organometallic complexes, have been difficult to work with because they degrade quickly and are not readily taken up by cells. Protein cages have attracted interest for drug delivery for some time, since they can retain the activity and stability of materials encapsulated in them. Recent reports have also demonstrated the ability to reduce the cytotoxicity and increase the uptake of metal nanoparticles by encapsulating them in a cage of ferritin, an iron-storage protein. "Therefore we chose to investigate whether ferritin can be used as an intracellular CO release molecule delivery carrier," explain Ueno and his team in their report.
The researchers used crystal structure analysis and confocal imaging to study the structure ruthenium carbonyl complexes encapsulated in the ferritin cage, as well as the uptake by cells. The researchers successfully developed a ferritin encapsulating ruthenium-carbonyl complexes that increased the uptake of CO releasing molecules and the amount of CO released inside the cell. Their composite also allowed slow CO release, which is important for activating processes inside cells.
Carbon monoxide is known to activate the nuclear factor κB in the presence of a certain tumour necrosis (cell injuring) factor. The researchers studied assays of mammalian cells incubated with the ferritin encapsulated ruthenium-carbonyl complexes. Optimising the composite yielded a tenfold increase in activation of nuclear factor κB and highlighted the benefits of slow CO release.
The researchers conclude their report, "Composites of ferritin and carbon monoxide releasing molecules can be applied as chemical tools to conduct extensive research on CO gas biology for clinical applications."
Background
Protein cages
Structures forming a cage from several weakly bonded protein subunits have attracted interest for encapsulating metal complexes and nanoparticles with catalytic, magnetic and photonic functions for biomedical applications. They have also been used for drug delivery.
The ability to deliver iron oxide nanoparticles into living cells using ferritin cages has already been demonstrated. Ferritin also readily undergoes cell surface endocytosis, a process by which cells recognize absorb molecules, and reduces the cytotoxicity of encapsulated particles.
CO release mechanisms
The intracellular signalling functions of carbon monoxide produce important cytotoxic effects against inflammation, proliferation (cell growth) and apoptosis (cell death).
The mechanism for the release of CO from ruthenium-carbonyl complexes inside molecules is understood as a ‘ligand exchange'. The bonds in the complex take the form of ligands, molecules that bind through the donation of electron pairs. Other electron-donating molecules inside the cell can exchange with the ligand releasing the carbon monoxide. The encapsulating ferritin cage can affect the ligand accessibility of these ligands, which in turn can affect the release of CO from the complex into cell.
The effect of release rate
The CO releasing molecule ferritin complexes devised by the authors released CO with a half-life 18 times slower than that in previous studies. In addition, previous reported ruthenium-carbonyl complexes did not show the concentration-dependence activation for nuclear factor κB. The researchers conclude from this that slow release is more crucial for activation by CO than the uptake ratio of CO releasing molecules by cells.
Reference
Kenta Fujita, Yuya Tanaka, Takeya Sho, Shuichi Ozeki, Satoshi Abe, Tatsuo Hikage, Takahiro Kuchimaru, Shinae Kizaka-Kondoh, and Takafumi Ueno. "Intracellular CO release from composite of ferritin and ruthenium carbonyl complexes." Journal of the American Chemical Society, Published Online (28 Octoer 2014); doi: 10.1021/ja508938f
####
About Tokyo Institute of Technology
As one of Japan’s top universities, Tokyo Institute of Technology seeks to contribute to civilization, peace and prosperity in the world, and aims at developing global human capabilities par excellence through pioneering research and education in science and technology, including industrial and social management. To achieve this mission, we have an eye on educating highly moral students to acquire not only scientific expertise but also expertise in the liberal arts, and a balanced knowledge of the social sciences and humanities, all while researching deeply from basics to practice with academic mastery. Through these activities, we wish to contribute to global sustainability of the natural world and the support of human life.
For more information, please click here
Contacts:
Yukiko Tokida
Asuka Suzuki
Center for Public Affairs and Communications
Tokyo Institute of Technology
2-12-1, Ookayama, Meguro-ku, Tokyo 152-8550, Japan
Tel: +81-3-5734-2975
Fax: +81-3-5734-3661
Copyright © Tokyo Institute of Technology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








