Home > Press > On the Road to Artificial Photosynthesis: Berkeley Lab Study Reveals Key Catalytic Factors in Carbon Dioxide Reduction
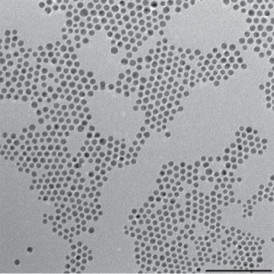 |
| This TEM shows gold–copper bimetallic nanoparticles used as catalysts for the reduction of carbon dioxide, a key reaction for artificial photosynthesis. |
Abstract:
The excessive atmospheric carbon dioxide that is driving global climate change could be harnessed into a renewable energy technology that would be a win for both the environment and the economy. That is the lure of artificial photosynthesis in which the electrochemical reduction of carbon dioxide is used to produce clean, green and sustainable fuels. However, finding a catalyst for reducing carbon dioxide that is highly selective and efficient has proven to be a huge scientific challenge. Meeting this challenge in the future should be easier thanks to new research results from Berkeley Lab.
On the Road to Artificial Photosynthesis: Berkeley Lab Study Reveals Key Catalytic Factors in Carbon Dioxide Reduction
Berkeley, CA | Posted on September 25th, 2014Peidong Yang, a chemist with Berkeley Lab's Materials Sciences Division, led a study in which bimetallic nanoparticles of gold and copper were used as the catalyst for the carbon dioxide reduction. The results experimentally revealed for the first time the critical influence of the electronic and geometric effects in the reduction reaction.
"Acting synergistically, the electronic and geometric effects dictate the binding strength for reaction intermediates and consequently the catalytic selectivity and efficiency in the electrochemical reduction of carbon dioxide," Yang says. "In the future, the design of carbon dioxide reduction catalysts with good activity and selectivity will require the careful balancing of these two effects as revealed in our study."
Yang, who also holds appointments with the University of California (UC) Berkeley and the Kavli Energy NanoSciences Institute at Berkeley, is a leading authority on nanoparticle phenomena. His most recent research has focused on nanocatalysts fashioned from metal alloys rather than a single metal such as gold, tin or copper.
"By alloying, we believe we can tune the binding strength of intermediates on a catalyst surface to enhance the reaction kinetics for the carbon dioxide reduction," he says. "Nanoparticles provide an ideal platform for studying this effect because, through appropriate synthetic processes, we can access a wide range of compositions, sizes and shapes, allowing for a deeper understanding of catalyst performance through precise control of active sites."
In addition, Yang says, nanoparticle as catalysts have high surface-to-volume and surface-to-mass ratios that are advantageous for achieving high catalytic activity. For this new study, uniform gold-copper bimetallic nanoparticles with different compositions were assembled into ordered monolayers then observed during carbon dioxide reduction.
"The ordered monolayers served as a well-defined platform that enabled us to better understand their fundamental catalytic activity in carbon dioxide reduction," Yang says. "Based on our observations, the activity of the gold-copper bimetallic nanoparticles can be explained in terms of the electronic effect, in which the binding of intermediates can be tuned using different surface compositions, and the geometric effect, in which the local atomic arrangement at the active site allows the catalyst to deviate from the scaling relation."
The effects Yang and his colleagues observed for gold-copper bimetallic nanoparticles should hold true for other carbon dioxide reduction catalysts as well.
"We expect the effects we observed to be universal for a wide range of catalysts, as evidenced in other areas of catalysis such as the hydrogen evolution and oxygen reduction reactions," says Dohyung Kim, a member of Yang's research group and a collaborator in this study. "The factors we have identified are based on the solid concept of electrocatalysis."
Knowing the influence of the electronic and geometric effects makes it possible to deduce how intermediate products in the reduction of carbon dioxide, such as carboxylic acid and carbon monoxide, will interact with the surface of a newly proposed catalyst and thereby provide the means for predicting the catalyst's performance. Coupled with the exceptional structuring of active catalytic sites made possible by the use of nanoparticles, the path is paved, Yang and his colleagues believe, for unprecedented improvements in electrochemical carbon dioxide reduction.
"My group is now using the insights gained from this study in the design of next generation carbon dioxide reduction catalysts," Yang says.
####
For more information, please click here
Contacts:
Lynn Yarris
510-486-5375
Copyright © DOE/Lawrence Berkeley National Laboratory
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() For more about the research of Peidong Yang go here:
For more about the research of Peidong Yang go here:
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Environment
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Onion-like nanoparticles found in aircraft exhaust May 14th, 2025
Energy
![]() Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
Sensors innovations for smart lithium-based batteries: advancements, opportunities, and potential challenges August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Solar/Photovoltaic
![]() Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
Spinel-type sulfide semiconductors to operate the next-generation LEDs and solar cells For solar-cell absorbers and green-LED source October 3rd, 2025
![]() KAIST researchers introduce new and improved, next-generation perovskite solar cell November 8th, 2024
KAIST researchers introduce new and improved, next-generation perovskite solar cell November 8th, 2024
![]() Groundbreaking precision in single-molecule optoelectronics August 16th, 2024
Groundbreaking precision in single-molecule optoelectronics August 16th, 2024
![]() Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
Development of zinc oxide nanopagoda array photoelectrode: photoelectrochemical water-splitting hydrogen production January 12th, 2024
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








