Home > Press > Diamond film possible without the pressure: Rice University, Russian researchers lay out rules for ultrathin 'diamane'
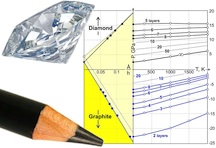 |
| The phase diagram developed by scientists at Rice University and in Moscow describes the conditions necessary for the chemical creation of thin films of diamond from stacks of single-atomic-layer graphene. Credit: Pavel Sorokin/Technological Institute for Superhard and Novel Carbon Materials |
Abstract:
Perfect sheets of diamond a few atoms thick appear to be possible even without the big squeeze that makes natural gems.
Diamond film possible without the pressure: Rice University, Russian researchers lay out rules for ultrathin 'diamane'
Houston, TX | Posted on February 3rd, 2014Scientists have speculated about it and a few labs have even seen signs of what they call diamane, an extremely thin film of diamond that has all of diamond's superior semiconducting and thermal properties.
Now researchers at Rice University and in Russia have calculated a "phase diagram" for the creation of diamane. The diagram is a road map. It lays out the conditions - temperature, pressure and other factors - that would be necessary to turn stacked sheets of graphene into a flawless diamond lattice.
In the process, the researchers determined diamane could be made completely chemically, with no pressure at all, under some circumstances.
The team led by Rice theoretical physicist Boris Yakobson and Pavel Sorokin, a former postdoctoral associate at Rice and now a senior researcher at the Technological Institute for Superhard and Novel Carbon Materials in Moscow, reported results in the American Chemical Society journal Nano Letters.
"Diamanes have a wide potential range of application," Sorokin said. "They can be applied as very thin, dielectric hard films in nanocapacitors or mechanically stiff, nanothick elements in nanoelectronics. Also, diamanes have potential for application in nano-optics.
"The possibility of obtaining such a quasi-two-dimensional object is intriguing, but available experimental data prevents the expectation of its fabrication using traditional methods. However, the 'bottom-up' approach proposed by Richard Feynman allows the fabrication of diamanes from smaller objects, such as graphene."
The researchers built computer models to simulate the forces applied by every atom involved in the process. That includes the graphene, the single-atom-thick form of carbon and one of the strongest substances in the universe, as well as the hydrogen (or, alternately, a halogen) that promotes the reaction.
Conditions, they learned, need to be just right for a short stack of graphene pancakes to collapse into a diamond matrix - or vice versa - via chemistry.
"A phase diagram shows you which phase dominates the ground state for each pressure and temperature," Yakobson said. "In the case of diamane, the diagram is unusual because the result also depends on thickness, the number of layers of graphene. So we have a new parameter."
Hydrogen isn't the only possible catalyst, he said, but it's the one they used in their calculations. "When the hydrogen attacks, it takes one electron from a carbon atom in graphene. As a result, a bond is broken and another electron is left hanging on the other side of the graphene layer. It's now free to connect to a carbon atom on the adjacent sheet with little or no pressure.
"If you have several layers, you get a domino effect, where hydrogen starts a reaction on top and it propagates through the bonded carbon system," he said. "Once it zips all the way through, the phase transition is complete and the crystal structure is that of diamond."
Yakobson said the paper doesn't cover a possible deal-breaker. "The conversion from one phase to another starts from a small seed, a nucleation site, and in this process there's always what is called a nucleation barrier. We don't calculate that here." He said carbon normally prefers to be graphite (the bulk form of carbon used as pencil lead) rather than diamond, but a high nucleation barrier prevents diamond from making the transition.
"Thermodynamically, an existing diamond should become graphite, but it doesn't happen for exactly this reason," Yakobson said. "So sometimes it's a good thing. But if we want to make flat diamond, we need to find ways to circumvent this barrier."
He said the manufacture of synthetic diamond, which was first reliably made in the 1950s, requires very high pressures of about 725,000 pounds per square inch. Manufactured diamonds are used in hardened tools for cutting, as abrasives and even as high-quality gemstones grown via techniques that simulate the temperatures and pressures found deep in Earth, where natural diamond is forged.
Diamond films are also routinely made via chemical vapor deposition, "but they're always very poor quality because they're polycrystalline," Yakobson said. "For mechanical purposes, like very expensive sandpaper, they're perfect. But for electronics, you would need high quality for it to serve as a wide-band gap semiconductor."
The paper's lead author is Alexander Kvashnin, a former visiting student at Rice and a graduate student at the Moscow Institute of Physics and Technology (MIPT). Co-author Leonid Chernozatonskii is a researcher at the Emanuel Institute of Biochemical Physics at the Russian Academy of Sciences, Moscow. Sorokin holds appointments at MIPT and the National University of Science and Technology, Moscow. Yakobson is Rice's Karl F. Hasselmann Professor of Mechanical Engineering and Materials Science, a professor of chemistry and a member of the Richard E. Smalley Institute for Nanoscale Science and Technology.
The U.S. Air Force Office of Scientific Research, the U.S. Office of Naval Research, the Ministry of Education and Science of the Russian Federation and the Russian Foundation for Basic Research supported the study.
####
About Rice University
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation's top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,708 undergraduates and 2,374 graduate students, Rice's undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice has been ranked No. 1 for best quality of life multiple times by the Princeton Review and No. 2 for "best value" among private universities by Kiplinger's Personal Finance. To read "What they're saying about Rice," go to tinyurl.com/AboutRiceU.
Follow Rice News and Media Relations via Twitter @RiceUNews
For more information, please click here
Contacts:
Mike Williams
713-348-6728
Copyright © Rice University
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Materials/Metamaterials/Magnetoresistance
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
![]() Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
Researchers unveil a groundbreaking clay-based solution to capture carbon dioxide and combat climate change June 6th, 2025
![]() A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
A 1960s idea inspires NBI researchers to study hitherto inaccessible quantum states June 6th, 2025
![]() Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Institute for Nanoscience hosts annual proposal planning meeting May 16th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals/White papers/Posters
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Military
![]() Quantum engineers ‘squeeze’ laser frequency combs to make more sensitive gas sensors January 17th, 2025
Quantum engineers ‘squeeze’ laser frequency combs to make more sensitive gas sensors January 17th, 2025
![]() Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
Chainmail-like material could be the future of armor: First 2D mechanically interlocked polymer exhibits exceptional flexibility and strength January 17th, 2025
![]() Single atoms show their true color July 5th, 2024
Single atoms show their true color July 5th, 2024
![]() NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
NRL charters Navy’s quantum inertial navigation path to reduce drift April 5th, 2024
Research partnerships
![]() Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
Lab to industry: InSe wafer-scale breakthrough for future electronics August 8th, 2025
![]() HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
HKU physicists uncover hidden order in the quantum world through deconfined quantum critical points April 25th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








