Home > Press > Living cells behave as fluid-filled sponges
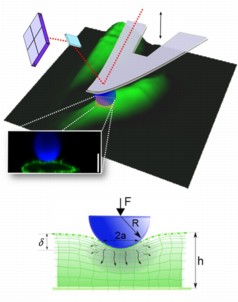 |
| Atomic Force Microscopy (AFM) microindentation enables investigation of the dynamic mechanical properties of living cells. Schematic of an AFM cantilever with a spherical tip indenting a cell. The image of the cells (green) is a 3D reconstruction from AFM topographical data. A confocal microscopy image shows the cell profile as the cell is deformed by a spherical bead (blue). The cell membrane is revealed using a fluorescent protein (shown in green) and a fluorescent bead is attached to the cantilever (in blue). Scale bar =10 μm. Below the profile image, a schematic diagram illustrates the indentation of a fluid-filled sponge (poroelastic material). Rapid indentation of the cellular material induces pressurisation of the interstitial fluid leading to its movement out of the compressed area. As the fluid redistributes, the pore pressure diminishes and the reaction force decreases. |
Abstract:
Animal cells behave like fluid-filled sponges in response to being mechanically deformed according to new research published in Nature Materials.
Scientists from the London Centre for Nanotechnology at UCL have shown that animal cells behave according to the theory of ‘poroelasticity' when mechanically stimulated in a way similar to that experienced in organs within the body. The results indicate that the rate of cell deformation in response to mechanical stress is limited by how quickly water can redistribute within the cell interior.
Living cells behave as fluid-filled sponges
London, UK | Posted on January 7th, 2013Poroelasticity was originally formulated to describe the behaviour of water-saturated soils and has important applications in the fields of rock engineering and petro-physics. It is commonly used in the petroleum industry. Poroelastic models describe cells as being analogous to fluid-filled sponges. Indeed, cells are constituted of a sponge-like porous elastic matrix (comprising the cytoskeleton, organelles, and macromolecules) bathed in an interstitial fluid (the cytosol). In this analogy, the rate at which the fluid-filled sponge can be deformed is limited by how fast internal water can redistribute within the sponge in response to deformation. This rate is dictated by three parameters: the stiffness of the sponge matrix, the size of the pores within the sponge matrix, and the viscosity of the interstitial fluid.
To study cellular responses, LCN scientists used cell-sized levers to apply rapid well-controlled deformations on the cell surface and monitored the temporal response of cells to these deformations. Close examination of the experimental results revealed that the rate of cellular deformation was limited by how rapidly water could redistribute within the cell interior. Experimental measurements indicated that this sponge-like behaviour of cells likely occurs during normal function of organs such as the lungs and the cardiovascular system.
Emad Moeendarbary, lead author of the paper from the LCN said:
"In the cardiovascular system, some tissues encounter extreme mechanical conditions. Heart valves can typically withstand seven-fold increases in their length in less than one second. The poroelastic nature of cells may allow them to behave similarly to shock absorbers when exposed to these extreme mechanical conditions."
To experimentally verify the fluid-sponge model, researchers manipulated the size of the cellular pores using chemical and genetic tools and showed that the rate of cellular deformation was affected by the pore size, as suggested by the theory of poroelasticity.
Guillaume Charras, senior co-author of the paper from the LCN said: "Cells can detect the mechanical forces they are subjected to and modify their behaviour accordingly. How changes in the mechanical environment are converted into biochemical information that the cell can interpret remains unknown. A better understanding of the physics of the cellular material is a first step towards formulating possible mechanisms through which this could occur."
####
For more information, please click here
Contacts:
London Centre for Nanotechnology
17-19 Gordon Street
London WC1H 0AH
tel: +44 (0)20 7679 0604
fax: +44 (0)20 7679 0595
Copyright © London Centre for Nanotechnology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() Journal link: Nature Materials, doi:10.1038/nmat3517:
Journal link: Nature Materials, doi:10.1038/nmat3517:
| Related News Press |
Imaging
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
![]() Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
Simple algorithm paired with standard imaging tool could predict failure in lithium metal batteries August 8th, 2025
![]() First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
First real-time observation of two-dimensional melting process: Researchers at Mainz University unveil new insights into magnetic vortex structures August 8th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








