Home > Press > Berkeley Lab Scientists Develop New Tool for the Study of Spatial Patterns in Living Cells: Golden Membranes Pave the Way for a Better Understanding of Cancer and the Immune System
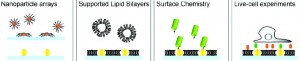 |
| Schematic shows gold nanoparticle arrays embedded into a supported lipid bilayer membrane then selectively labeled with specific surface chemistry properties to study living cells that are bound to the nanoparticles and/or lipid bilayer. (Groves, et. al) |
Abstract:
Football has often been called "a game of inches," but biology is a game of nanometers, where spatial differences of only a few nanometers can determine the fate of a cell - whether it lives or dies, remains normal or turns cancerous. Scientists with the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a new and better way to study the impact of spatial patterns on living cells.
Berkeley Lab Scientists Develop New Tool for the Study of Spatial Patterns in Living Cells: Golden Membranes Pave the Way for a Better Understanding of Cancer and the Immune System
Berkeley, CA | Posted on November 1st, 2011Berkeley Lab chemist Jay Groves led a study in which artificial membranes made up of a fluid bilayer of lipid molecules were embedded with fixed arrays of gold nanoparticles to control the spacing of proteins and other cellular molecules placed on the membranes. This provided the researchers with an unprecedented opportunity to study how the spatial patterns of chemical and physical properties on membrane surfaces influence the behavior of cells.
"The gold nanoparticles are similar to the size of a single protein molecule, which gets us to a scale we couldn't really access before," says Groves. "As the first example of a biological membrane platform that combines fixed nanopatterning with the mobility of fluid lipid bilayers, our technique represents an important improvement over previous patterning methods."
Groves holds joint appointments with Berkeley Lab's Physical Biosciences Division and the University of California (UC) Berkeley's Chemistry Department, and is a Howard Hughes Medical Institute (HHMI) investigator. He is the corresponding author of a paper that reports these results in the journal Nano Letters. The paper is titled "Supported Membranes Embedded with Fixed Arrays of Gold Nanoparticles."
Spatial patterning of chemical and physical properties on artificial membranes of lipid bilayers is a time-tested way to study the behavior of cultured biological cells. Natural lipid bilayer membranes surround virtually all living cells as well as many of the structures inside the cell including the nucleus. These membranes provide a barrier that restrains the movement of proteins and other cellular molecules, penning them into their proper locations and preventing them from moving into areas where they do not belong. Past spatial patterning efforts on artificial membranes have been done on an all-or-nothing basis - proteins placed on a membrane either had complete mobility or were fixed in a static position.
"Immobile patterning intrinsically defeats any cellular process that naturally involves movement," Groves says. "On the other hand we need to be able to impose some fixed barriers in order to manipulate membranes in really novel ways."
Groves is a recognized leader in the development of unique "supported" synthetic membranes that are constructed out of lipids and assembled onto a substrate of solid silica. He and his group have used these supported membranes to demonstrate that living cells not only interact with their environment through chemical signals but also through physical force.
"We call our approach the spatial mutation strategy because molecules in a cell can be spatially re-arranged without altering the cell in any other way," he says.
However, until now Groves and his group were unable to get to the tens of nanometers length-scales that they can now reach by embedding their supported membranes with gold nanoparticles.
"Our new membranes provide a hybrid interface consisting of mobile and immobile components with controlled geometry," Groves says. "Proteins or other cellular molecules can be associated with the fluid lipid component, the fixed nanoparticle component, or both."
The gold nanoparticle arrays were patterned through a self-assembly process that provides controllable spacing between particles in the array in the important range of 50 to 150 nanometers. The gold nanoparticles themselves measure about five to seven nanometers in diameter.
Groves and his team successfully tested their hybrid membranes on a line of breast cancer cells known as MDA-MB-231 that is highly invasive. With their hybrid membranes, the team demonstrated that in the absence of cell adhesion molecules, the membrane remained essentially free of the cancer cells, but when both the nanoparticles and the lipid were functionalized with molecules that promote cell adhesion, the cancer cells were found all over the surface.
Groves and his research group are now using their gold nanoparticle membranes to study both cancer metastasis and T cell immunology. They expect to report their results soon.
Co-authoring the Nano Letters paper with Groves were Theobald Lohmuller, Sara Triffo, Geoff O'Donoghue, Qian Xu and Michael Coyle. This research was supported by the DOE Office of Science.
####
About Berkeley Lab
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
For more information, please click here
Contacts:
Lynn Yarris
(510) 486-5375
Copyright © Berkeley Lab
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related Links |
![]() For more on the research of Jay Groves visit the Website at:
For more on the research of Jay Groves visit the Website at:
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() "Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
"Nanoreactor" cage uses visible light for catalytic and ultra-selective cross-cycloadditions October 3rd, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
Single-atom catalysts change spin state when boosted by a magnetic field June 4th, 2025
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Laboratories
![]() Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Researchers develop molecular qubits that communicate at telecom frequencies October 3rd, 2025
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Tools
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Japan launches fully domestically produced quantum computer: Expo visitors to experience quantum computing firsthand August 8th, 2025
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








