Home > Press > Seeing the Quantum in Chemistry: JILA Scientists Control Chemical Reactions of Ultracold Molecules
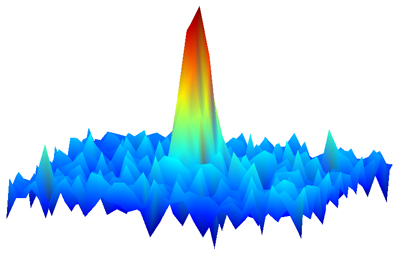 |
| One of the first-ever images of a molecular gas in which each molecule is in its lowest possible energy state. The gas has just been released from a trap created by lasers. The molecules are near absolute zero, a temperature at which quantum properties reign. The image – made by detecting the absorption of laser light by the molecules -- reveals their spatial distribution, with density indicated by peak height and false color. The fact that such an image can be created indicates the molecular quantum gas is dense enough to enable scientists to observe novel interactions among the molecules. Credit: D. Wang/JILA |
Abstract:
Physicists at JILA have for the first time observed chemical reactions near absolute zero, demonstrating that chemistry is possible at ultralow temperatures and that reaction rates can be controlled using quantum mechanics, the peculiar rules of submicroscopic physics.
Seeing the Quantum in Chemistry: JILA Scientists Control Chemical Reactions of Ultracold Molecules
Gaithersburg, MD | Posted on February 11th, 2010The new results and techniques, described in the Feb. 12 issue of Science,* will help scientists understand previously unknown aspects of how molecules interact, a key to advancing biology, creating new materials, producing energy and other research areas. The new JILA work also will aid studies of quantum gases (in which particles behave like waves) and exotic physics spanning the quantum and macroscopic worlds. It may provide practical tools for "designer chemistry" and other applications such as precision measurements and quantum computing.
JILA is a joint institute of the National Institute of Standards and Technology (NIST) and the University of Colorado at Boulder. A NIST theorist at the Joint Quantum Institute, a collaborative venture of NIST and the University of Maryland, also contributed to the research.
"It's perfectly reasonable to expect that when you go to the ultracold regime there would be no chemistry to speak of," says NIST physicist Deborah Jin, leader of one JILA group involved in the experiments. "This paper says no, there's a lot of chemistry going on."
"We are observing a new fundamental aspect of chemistry - it gives us a new ‘knob' to understand and control reactions," adds NIST physicist Jun Ye, leader of the second JILA group involved in the research.
The Science paper is a follow-up to the same research team's 2008 report of the first high-density gas of stable, strongly interacting ultracold molecules, each consisting of two different atoms bonded together (see www.nist.gov/public_affairs/releases/ultracold_polar_molecules.html).
Ultracold molecules are a hot research area because they may offer more diverse insights and applications than ultracold atoms, which scientists have deftly manipulated for more than 20 years.
Scientists have long known how to control the internal states of molecules, such as their rotational and vibrational energy levels. In addition, the field of quantum chemistry has existed for decades to study the effects of the quantum behavior of electrons and nuclei—constituents of molecules. But until now scientists have been unable to observe direct consequences of quantum mechanical motions of whole molecules on the chemical reaction process. Creating simple molecules and chilling them almost to a standstill makes this possible by presenting a simpler and more placid environment that can reveal subtle, previously unobserved chemical phenomena.
By precisely controlling the ultracold molecules' internal states—electronic energy levels, vibrations, rotations and nuclear spin (or angular momentum)—while also controlling the molecular motions at the quantum level, JILA scientists can study how the molecules scatter or interact with each other quantum mechanically. They were able to observe how the quantum effects of the molecule as a whole dictate reactivity. This new window into molecular behavior has allowed the observation of long-range interactions in which quantum mechanics determines whether two molecules should come together to react or stay apart. Thus the JILA work pushes the field in new directions and expands the standard conception of chemistry.
The JILA quantum chemistry experiments were performed with a gas containing up to 1 trillion molecules per cubic centimeter at temperatures of a few hundred billionths of a Kelvin (nanokelvins) above absolute zero (minus 273 degrees Celsius or minus 459 degrees Fahrenheit). Each molecule consists of one potassium atom and one rubidium atom. The molecules have a negative electric charge on the potassium side and a positive charge on the rubidium side, so they can be controlled with electric fields.
By measuring how many molecules are lost over time from a gas confined inside a laser-based optical trap, at different temperatures and under various other conditions, the JILA team found evidence of heat-producing chemical reactions in which the molecules must have exchanged atoms, broken chemical bonds, and forged new bonds. Theoretical calculations of long-range quantum effects agree with the experimental observations.
In conventional chemistry at room temperature, molecules may collide and react to form different compounds, releasing heat. In JILA's ultracold experiments, quantum mechanics reigns and the molecules spread out as ethereal rippling waves instead of acting as barbell-like solid particles. They do not collide in the conventional sense. Rather, as their quantum mechanical wave properties overlap, the molecules sense each other from as much as 100 times farther apart than would be expected under ordinary conditions. At this distance the molecules either scatter from one another or, if quantum conditions are right, swap atoms. Scientists expect to be able to control long-range interactions by creating molecules with specific internal states and "tuning" their reaction energies with electric and magnetic fields.
The JILA team produced a highly dense molecular gas and found that, although molecules move slowly at ultralow temperatures, reactions can occur very quickly. However, reactions can be suppressed using quantum mechanics. For instance, a cloud of molecules in the lowest-energy electronic, vibrational and rotational states reacts differently if the nuclear spins of some molecules are flipped. If a cloud of molecules is divided 50/50 into two different nuclear spin states, reactions proceed 10 to 100 times faster than if all molecules possess the same spin state. Thus, by purifying the gas (by preparing all molecules in the same spin state), scientists can deliberately suppress reactions.
The JILA experimental team attributes these results to the fact the molecules are fermions, one of two types of quantum particles found in nature. (Bosons are the second type.) Two identical fermions cannot be in the same place at the same time. This quantum behavior of fermions manifests as a suppression of the chemical reaction rate in the ultralow temperature gas. That is, molecules with identical nuclear spins are less likely to approach each other and react than are particles with opposite spins.
The JILA research is supported by NIST, the National Science Foundation and the Department of Energy.
As a non-regulatory agency of the U.S. Department of Commerce, NIST promotes U.S. innovation and industrial competitiveness by advancing measurement science, standards and technology in ways that enhance economic security and improve our quality of life.
* S. Ospelkaus, K.K. Ni, D. Wang, M.H.G. de Miranda, B. Neyenhuis, G. Quéméner, P.S. Julienne, J.L. Bohn, D.S. Jin, and J. Ye. 2010. Quantum-State Controlled Chemical Reactions of Ultracold KRb Molecules. Science. Feb. 12.
####
About NIST
From automated teller machines and atomic clocks to mammograms and semiconductors, innumerable products and services rely in some way on technology, measurement, and standards provided by the National Institute of Standards and Technology.
Founded in 1901, NIST is a non-regulatory federal agency within the U.S. Department of Commerce. NIST's mission is to promote U.S. innovation and industrial competitiveness by advancing measurement science, standards, and technology in ways that enhance economic security and improve our quality of life.
For more information, please click here
Contacts:
Laura Ost
(303) 497-4880
University of Colorado Contact:
Peter Caughey
(303) 492-4007
Copyright © NIST
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
Chemistry
![]() Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
Projecting light to dispense liquids: A new route to ultra-precise microdroplets January 30th, 2026
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Govt.-Legislation/Regulation/Funding/Policy
![]() Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
Metasurfaces smooth light to boost magnetic sensing precision January 30th, 2026
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Discoveries
![]() From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
From sensors to smart systems: the rise of AI-driven photonic noses January 30th, 2026
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Alliances/Trade associations/Partnerships/Distributorships
![]() Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
Chicago Quantum Exchange welcomes six new partners highlighting quantum technology solutions, from Chicago and beyond September 23rd, 2022
![]() University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
University of Illinois Chicago joins Brookhaven Lab's Quantum Center June 10th, 2022
Quantum nanoscience
![]() Beyond silicon: Electronics at the scale of a single molecule January 30th, 2026
Beyond silicon: Electronics at the scale of a single molecule January 30th, 2026
![]() MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
MXene nanomaterials enter a new dimension Multilayer nanomaterial: MXene flakes created at Drexel University show new promise as 1D scrolls January 30th, 2026
![]() ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
ICFO researchers overcome long-standing bottleneck in single photon detection with twisted 2D materials August 8th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








