Home > Press > A Revolutionary Approach to Tissue Engineering
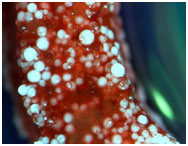 |
| Microsphere (white spheres) sintering based scaffolds for bone tissue engineering, showing calcium deposition by cells (red) |
Abstract:
Nanotechnology is leading to advancements in Tissue Engineering research that makes use of biomedical scaffolds to reduce recovery times and offer new health solutions.
A Revolutionary Approach to Tissue Engineering
Hoboken, NJ | Posted on October 29th, 2009Tissue Engineering is an emerging multidisciplinary field involving biology, medicine, and engineering that is likely to revolutionize the ways we improve the health and quality of life for millions of people by restoring, maintaining, or enhancing tissue and organ function. It involves the use of a combination of cells, engineering and material methods, including suitable biochemical and chemical factors to improve or replace biological functions. Most often used in cases of patient trauma, it is critical in repairing lost tissue function, assisting the healing process, cosmetic applications, as well as the prevention and risk mitigation of infections. These devices are typically created by using a biomaterial scaffold which allows cells to attach and reorganize to form functional tissue by proliferating, synthesizing extracellular matrix, and migrating along the implant path.
Dr. Xiaojun Yu and Dr. Hongjun Wang of the Department of Chemistry, Chemical Biology and Biomedical Engineering at Stevens Institute of Technology are working with several different types of tissue engineering; including skin, bone, nerve and cardiac muscle. They utilize principles of nanotechnology and a "bottom-up" philosophy that may result in exponentially faster recovery times and improve patient integration, as well as enable the introduction of 3-dimensional multilayer tissue formation due to advanced scaffold techniques, which have been developed in their respective labs. This improved scaffold provides a better location for cells to attach and regenerate, and because it is biodegradable, only native cells remain. With the potential to help burn victims, congestive heart failure patients, peripheral nerve injuries, spinal cord injuries, broken bones and more, the medical and industrial implications of their work are tremendous.
How is Tissue Engineering research unique at Stevens?
The traditional method of Tissue Engineering involves implanting a scaffold with cultured cells in a specific location. The difficulties in standard development methods result from the seeding and culture of cells in a preformatted porous scaffold, which is often inadequate in restoring the lost function of diseased tissues. This is due in part to the lack of structural integrity and non-uniformity of the porous scaffold implants. With current skin grafting techniques, the procedure can take up to three weeks. Comparatively, the research from Dr. Wang's lab has proved so useful that the skin grafting process can be shortened from three weeks to just one day.
Professor Wang has been studying this field since 1995, and believes the main limitation of traditional techniques stems from "the inability to make a complex tissue as a result of a ‘top-down' approach, leading to slow progress in tissue engineering." His research has resulted in the development of a "bottom-up" methodology, using nanotechnology to enable development of scaffolds that match the biological properties of the patient. Researchers have adopted the philosophies of other engineering disciplines, and are now focusing major research efforts on these improvements. The idea is that by working on the smallest level possible and building upward, they are more likely to develop a scaffold and cell cultures that match the native extra cellular matrix (ECM), that is, the complex structural entity surrounding and supporting cells found within mammalian tissues. Professor Wang highlights his skin grafting improvement goals:
"The repair and management of extensive burn wounds have long been a problem and served as a difficulty in conventional Tissue Engineering methods. Autografts are often used in burn treatment; however, insufficient supply and the secondary wounds created from harvesting autografts limit its applicability for extensive burns. Autologous tissue engineered skin substitutes (ATESS) are considered a promising alternative to autografts. They possess many of the properties of an autograft, such as wound protection, accelerated wound closure, high graft take, new skin regeneration, and not subject to immunorejection. For extensive burn treatment using ATESS, there are two unsolved and critical issues: 1) the in vitro time to create the skin substitutes should be as short as possible, allowing rapid wound closure; 2) the size of ATESS should be as large as possible to cover enough wounded area. Current tissue-engineering approaches require prolonged culture time and cannot produce large skin substitutes. Therefore, it is desirable to develop new approaches and strategies toward rapid production of autologous skin substitutes with up-scaling potential. The primary objective of our research is to develop a novel practical protocol for rapid creation of ATESS using a nanofiber-enabled layer-by-layer cell assembly approach. Our specific aims are: 1) in vitro preparation and characterization of skin substitutes using layer-by-layer assembly of skin cells, 2) in vivo determination of the effect of tissue engineered skin substitutes on the healing of acute full-thickness wounds in a mouse model."
With nerves, disruptions in the signal pathways often lead to lack of mobility and sensation, so the main goal is to develop scaffolding along the repair pathway of the nerve to facilitate new growth. Dr. Yu's research on nanofiber-based artificial nerve grafts explains further:
"Peripheral nerve injuries are common diseases that affect hundreds of thousands of patients every year. Severe nerve injuries result in the formation of a significant gap between the severed nerve stumps. When the gap is large (4 mm), autografts are commonly used as ‘bridges.' However, this is not ideal because the availability of autografts is limited, and the patient is exposed to the morbidity of a second surgical incision site. Until now, many approaches have been examined for enhancing peripheral nerve regeneration. However, none of them has performed better than autograft procedures, the 'gold standard' for repairing peripheral nerve injuries. One of the key projects in Dr.Yu's lab is the development of nanofiber based biodegradable nerve grafts pre-seeded with Schwann cells (supporting cells) in bioreactors for enhancing peripheral nerve regeneration to a level comparable to autografts. The bioreactor culture system will help fasten and improve Schwann cell growth and differentiation on the artificial nerve graft."
In the case of bone injury, the break or structural inefficiency is often localized at a specific point of impact. In these situations, Bone Regeneration techniques may be used in order to restore strength and functionality. In hard tissue trauma where the injury consists of large gaps, a bone grafting procedure is necessary. Polymeric nanofiber matrices are biologically similar to the native extracellular matrix architecture, and thus, have the potential to be used as bone grafts. Traditional methods of Tissue Engineering are restricted by the complex fabrication nature of these nanofibers into three-dimensional (3D) structures. This results in mechanical limitations of the scaffold, and insufficient properties for direct bone applications. "To overcome this, we have incorporated nanofibers onto biodegradable polymeric 3D scaffolds that have a novel spiral architecture," says Dr.Yu. These scaffolds have open geometries, large surface areas and an optimal porosity for cell migration, which better enable nutrient transport and cell penetration needed for successful repair of large bone defects. These scaffolds have the potential for universal applicability in the treatment of bone injuries.
In addition to bone regeneration techniques, Dr. Yu has researched methods to improve upon spinal fusion methods. Spinal fusion has historically been done by using metal-based apparatuses or grafts, which utilize allogenic or autogenic sources of bone, but ultimately immobilization with these metal rods has shown adjacent disc degeneration. We have developed a biphasic biodegradable polymeric scaffold that promotes the fusion of two adjacent vertebrae when situated in place of a defective IVD. This approach avoids the use of titanium or any metal implants thus decreasing the impact on the immune system and is biodegradable thus bypassing the complications of a permanent scaffold or construct.
Several common themes are prevalent among the different research specialties in Tissue Engineering at Stevens Institute of Technology. The first step is developing a better scaffold. Using electro-spun nanofibers, Dr. Yu and Dr. Wang are able to mimic the properties of collagen, one of the major components of a native tissue matrix. Recent developments in microfabrication technology enable fabrication of scaffolds on a micronanometer scale. Electrospinning, which is a high-voltage driven spinning technique, receives particular attention thanks to its ability to produce nanofibrous scaffolds, having not only similar dimensions as collagen fibrils in natural tissue matrix, but also various fiber attributes in spatial arrangement and mechanical properties. Scaffold improvements will lead to greater acceptance of cell growth and integration, which in turn leads to vastly improved recovery times.
Much of the success in the Tissue Engineering research can be attributed to the efforts of Stevens undergraduate, graduate and doctoral students. Students such as Chandra M. Valmikinathan, Jeckin D. Shah, Wei Chang, Harinder Bawa, Elizabeth Hagan, and Amanda L. Foley detail the opportunities for research.
Chandra M. Valmikinathan is an accomplished PhD candidate, who has worked with Professor Yu since 2005. His research focus pertains to biomaterial based approaches to repair and regenerate lost tissues. Chandra's thesis project, "The use of electrospun nanofiber based scaffolds for peripheral nerve regeneration," is integrated with the schools' "bottom-up" philosophy, whereby nanotechnology enables researchers to improve the scaffold so that it resembles native tissue and is successfully accepted by the patient. He has also been involved in development of systems that release specific growth promoting agents such as growth factors, as well as systems that can release tumor killing drugs.
"Tissue Engineering involves a blend of several research fields, and the research environment in Stevens fosters collaboration," says Chandra. He chose Stevens because of the accomplished faculty, state-of-the-art facilities and proximity of many large biomedical companies.
Jeckin D. Shah received his Masters in Biomedical Engineering in 2007 and worked with Dr. Wang on research involving electrospinning methods to uniformly distribute osteoblasts over a scaffold. Uniform distribution of osteoblasts enables "control over the number of cells on that scaffold, ability to integrate cells within the scaffold and ability to regenerate bone tissue in a more natural state." He is also the founder of SPOC medical, a Stevens Technogenesis™ company originally conceived in Professor Vikki Hazelwood's Biomedical Senior Design class during the fall of 2004. Wei Chang is another graduate student in Professor Yu's lab who specializes in biomimic bone scaffolding.
Along with graduate students and doctoral candidates, the programs at Stevens heavily involve undergraduate students as key members of research projects. One such student is Harinder Bawa, whose work involves the "prevention of biofilm formation using biodegradable and biocompatible hydrogels." One of the major problems in implant surgery is the high risk of infection due to bacteria attaching to the surface of the implant prior to surgery. Professor Yu, Chandra and Harinder have designed a hydrogel that can be used to coat implants prior to surgery in a three to seven minute process which prevents the attachment of bacteria, formation of biofilm and improves patient integration.
Elizabeth Hagan is an undergraduate student of Professor Wang who holds a personal connection with the field of Biomedical Engineering. Diagnosed at birth with a rare bone condition in which calcium deposits and pieces of bone appear on the skin, Elizabeth is using her passion for science, math and biology to develop alternative therapies and help patients with similar conditions. Her research began as a summer 2009 Technogenesis™ Scholar in which she learned how to fabricate scaffolds for bone tissue engineering, understand how to evaluate the biomechanics of formed bone, and characterize the cellular response.
Amanda L. Foley is another undergraduate student working in Professor Wang's laboratory, whose research focuses on experimental ways to construct aligned scaffolds for electrospinning, in addition to constructing random scaffolds. These scaffolds are then constructed with cultured cells to build layers of skin tissues, with the ultimate goal of making strong enough tissues to be used on the human body. She has also become skilled in application of gel electrophoresis and polymerase chain reaction experiments.
Conclusion
Professors Yu and Wang are making strides in the advancement of Tissue Engineering research. The application of engineering techniques to biomedical procedures is vastly improving important aspects of this field, such as infection prevention and patient recovery. Professor Wang compared the application of nanotechnology and this "bottom-up" approach to the standard practices in Engineering, "For years engineers working with mechanical objects would work to build the most efficient, reliable and effective base unit, then build upwards." The idea of using nanotechnology to improve patient integration of scaffolding, cell, and grafting procedures has been revolutionary and is enabling new possibilities now and in the future.
In addition to providing research opportunities and health advancements, the nature of this field makes use of various Engineering disciplines, and serves as an ideal "academic bridge between the disciplines of Chemistry, Biology, and Biomedical Engineering," explains Dr. Philip Leopold, Professor & Department Director. Dr. Yu has been the recipient of several grants, including funding from the Arbeitsgemeinschaft für Osteosynthesefragen (AO) foundation for his research on biphasic constructs for spinal fusion. He is also funded by the Coulter Foundation; and by the National Institute of Health (NIH) for his peripheral nerve project. Dr. Wang has also received a grant from the NIH for his work involving development of skin grafts that promote rapid re-growth due to improved scaffolding on the bone tissue engineering frontier.
The unique and award winning research being done by these Professors, combined with the challenging course offerings, top rate facilities and devoted student body, provides for an excellent academic experience, and makes the department of Chemistry, Chemical Biology and Biomedical Engineering a highly respected authority on Tissue Engineering.
####
About Stevens Institute of Technology
The research enterprise includes academic research, national research centers, and cross-cutting research initiatives, with supporting organizations and infrastructure. Creative and entrepreneurial research is an integral part of the Stevens mission. Building Communities for Research in focused areas has been a top priority of the Institute Strategic Plan and the Office of the Provost. Such communities are bound by a common purpose to achieve something distinctive, cross over department, and school boundaries and are the cornerstone of the extended research enterprise, supported as a top institutional priority. The fundamental principles governing the research environment at Stevens are Inventiveness, Creativity and Entrepreneurship (ICE). Technogenesis, the learning environment practiced at Stevens, involves students, faculty and colleagues from government and industry working together from invention to market realization of technology. The Office of Research Enterprise provides incentives, support and encouragement in building and sustaining cross disciplinary clusters within such communities.
For more information, please click here
Contacts:
Doug Fabrizio
Phone: 201-216-8910
Copyright © Stevens Institute of Technology
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
| Related News Press |
News and information
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Possible Futures
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanomedicine
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
Cambridge chemists discover simple way to build bigger molecules – one carbon at a time June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Announcements
![]() Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
Decoding hydrogen‑bond network of electrolyte for cryogenic durable aqueous zinc‑ion batteries January 30th, 2026
![]() COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
COF scaffold membrane with gate‑lane nanostructure for efficient Li+/Mg2+ separation January 30th, 2026
Nanobiotechnology
![]() New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
New molecular technology targets tumors and simultaneously silences two ‘undruggable’ cancer genes August 8th, 2025
![]() New imaging approach transforms study of bacterial biofilms August 8th, 2025
New imaging approach transforms study of bacterial biofilms August 8th, 2025
![]() Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
Ben-Gurion University of the Negev researchers several steps closer to harnessing patient's own T-cells to fight off cancer June 6th, 2025
![]() Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
Electrifying results shed light on graphene foam as a potential material for lab grown cartilage June 6th, 2025
|
|
||
|
|
||
| The latest news from around the world, FREE | ||
|
|
||
|
|
||
| Premium Products | ||
|
|
||
|
Only the news you want to read!
Learn More |
||
|
|
||
|
Full-service, expert consulting
Learn More |
||
|
|
||








